药物研发
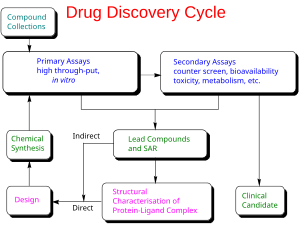
药物研发是指一旦通过药物发现确定了先导化合物,就将一种新的药物推向市场的过程。具体步骤包括对微生物和动物的临床前研究,申请监管机构批准(例如获得美国食品药品监督管理局批准)后即可为研究性新药启动人体临床试验,之后监管部门批准新药申请,这些流程都通过后,药物才能推向市场[1][2] 。
参考文献
编辑- ^ Taylor, David. The Pharmaceutical Industry and the Future of Drug Development. Issues in Environmental Science and Technology (Royal Society of Chemistry). 2015: 1–33 [2020-11-13]. ISBN 978-1-78262-189-8. doi:10.1039/9781782622345-00001. (原始内容存档于2021-04-28) (英语).
- ^ Strovel, Jeffrey; Sittampalam, Sitta; Coussens, Nathan P.; Hughes, Michael; Inglese, James; Kurtz, Andrew; Andalibi, Ali; Patton, Lavonne; Austin, Chris. https://www.ncbi.nlm.nih.gov/books/NBK92015/
|chapterurl=缺少标题 (帮助). Early Drug Discovery and Development Guidelines: For Academic Researchers, Collaborators, and Start-up Companies. Eli Lilly & Company and the National Center for Advancing Translational Sciences. July 1, 2016 [2020-11-13]. PMID 22553881. (原始内容存档于2020-04-19).