1,2-二𫫇英
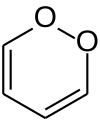
1,2-二𫫇英(英语:1,2-Dioxin)是一种杂环有机反芳香性[1]化合物,化学式为C4H4O2。它是1,4-二𫫇英的同分异构体。
| 1,2-二𫫇英 | |||
|---|---|---|---|
| |||
| |||
| IUPAC名 1,2-Dioxine 1,2-二氧杂环己熳 | |||
| 系统名 1,2-Dioxacyclohexa-3,5-diene 1,2-二氧杂环-3,5-己二烯 | |||
| 识别 | |||
| CAS号 | 289-87-2 | ||
| PubChem | 15559065 | ||
| ChemSpider | 10606250 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | VCZQYTJRWNRPHF-UHFFFAOYAH | ||
| 性质 | |||
| 化学式 | C4H4O2 | ||
| 摩尔质量 | 84.07 g·mol−1 | ||
| 相关物质 | |||
| 相关化学品 | 二苯并对二𫫇英 1,4-二𫫇英 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
由于它类似于过氧化物的性质,1,2-二𫫇英非常不稳定,仍未分离。根据计算,它会快速异构化成2-丁烯二醛。[2]有取代基的衍生物也非常不稳定,例如1,4-二苯基-2,3-苯并二𫫇英。[3]1990年,3,6-二(对甲苯基)-1,2-二𫫇英被误认为是第一个稳定的衍生物,[4]之后表明初始化合物不是1,2-二𫫇英的衍生物,而是一个热力学上更稳定的二酮。[5]
-
同分异构体1,2-二𫫇英(左)和1,4-二𫫇英(右)
-
不稳定的1,4-二苯基-2,3-苯并二𫫇英的结构
-
二𫫇英(1)和二酮形式(2)
参考文献
编辑- ^ Pelloni, Stefano; Faglioni, Francesco; Lazzeretti, Paolo. Parity violation energies of C4H4X2 molecules for X = O, S, Se, Te and Po. Molecular Physics. 2013-09-01, 111 (16-17): 2387–2391. ISSN 0026-8976. doi:10.1080/00268976.2013.794396.
- ^ Matsumoto, M. Product Class 1: 1,2-Dioxins and Benzo- and Dibenzo- Fused Derivatives. Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 16: Six-Membered Hetarenes with Two Identical Heteroatoms. Georg Thieme Verlag. 2014: 13 [2020-06-12]. ISBN 9783131718815. (原始内容存档于2022-03-27).
- ^ Smith, Jimmie P.; Schrock, Alan K.; Schuster, Gary B. Chemiluminescence of organic peroxides. Thermal generation of an o-xylylene peroxide. Journal of the American Chemical Society. 1982, 104 (4): 1041. doi:10.1021/ja00368a021..
- ^ Shine, Henry J.; Zhao, Da Chuan. Electron transfer to excited doublet states. Photoirradiation of 10-methylphenothiazine cation radical perchlorate in solutions of phenylacetylene and p-tolylacetylene in acetonitrile. The Journal of Organic Chemistry. 1990, 55 (13): 4086. doi:10.1021/jo00300a026..
- ^ Block, Eric; Shan, Zhixing; Glass, Richard S.; Fabian, Jürgen. Revised Structure of a Purported 1,2-Dioxin: A Combined Experimental and Theoretical Study. The Journal of Organic Chemistry. 2003, 68 (10): 4108–11. PMID 12737603. doi:10.1021/jo034305i.