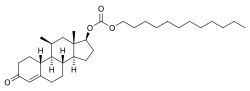
11β-甲基-19-去甲睾酮十二烷基碳酸酯
11β-甲基-19-去甲睾酮十二烷基碳酸酯[1](英语:11β-Methyl-19-nortestosterone 17β-dodecylcarbonate,药物开发代码:CDB-4754),简称11β-MNTDC 。是一种合成的口服蛋白同化甾类药物,为19-去甲睾酮的衍生物。由美国国家儿童健康与人类发育研究所(NICHD)所开发,目前尚未上市的药物[2][3][4][5]。其为雄激素酯衍生物,是11β-甲基-19-去甲睾酮(11β-MNT)与十二烷基碳酸形成的酯,同时也是11β-MNT的前体药物[2][3][4][5]。
 | |
| 临床资料 | |
|---|---|
| 其他名称 | 11β-MNTDC; CDB-4754 |
| 给药途径 | 口服, 肌肉注射 |
| 药物类别 | 雄激素; 蛋白同化甾类; 雄激素酯; 孕激素 |
| 识别信息 | |
| |
| CAS号 | 904901-01-5 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
| 化学式 | C32H52O4 |
| 摩尔质量 | 500.76 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
参考文献
编辑- ^ 两款男性口服避孕药已经通过了Ⅰ期临床试验. 上海医药. 2022, 43 (13): 58.
- ^ 2.0 2.1 Attardi BJ, Marck BT, Matsumoto AM, Koduri S, Hild SA. Long-term effects of dimethandrolone 17β-undecanoate and 11β-methyl-19-nortestosterone 17β-dodecylcarbonate on body composition, bone mineral density, serum gonadotropins, and androgenic/anabolic activity in castrated male rats. Journal of Andrology. 2011, 32 (2): 183–192. PMID 20798389. doi:10.2164/jandrol.110.010371 .
- ^ 3.0 3.1 Attardi BJ, Hild SA, Koduri S, Pham T, Pessaint L, Engbring J, et al. The potent synthetic androgens, dimethandrolone (7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone, do not require 5α-reduction to exert their maximal androgenic effects. The Journal of Steroid Biochemistry and Molecular Biology. October 2010, 122 (4): 212–218. PMC 2949447 . PMID 20599615. doi:10.1016/j.jsbmb.2010.06.009.
- ^ 4.0 4.1 Hild SA, Attardi BJ, Koduri S, Till BA, Reel JR. Effects of synthetic androgens on liver function using the rabbit as a model. Journal of Andrology. 2010, 31 (5): 472–481. PMC 2943539 . PMID 20378929. doi:10.2164/jandrol.109.009365.
- ^ 5.0 5.1 US 7820642,Blye RP, Kim HK,“Nandrolone 17β-carbonates”,发行于26 October 2010,指定于U.S. Department of Health and Human Services
- ^ Thirumalai A, Page ST. Recent Developments in Male Contraception. Drugs. January 2019, 79 (1): 11–20. PMID 30588563. S2CID 56895132. doi:10.1007/s40265-018-1038-8.
- ^ Second potential male birth control pill passes human safety tests.
- ^ Yuen F, Thirumalai A, Pham C, Swerdloff RS, Anawalt BD, Liu PY, Amory JK, Bremner WJ, Dart C, Wu H, Hull L, Blithe DL, Long J, Wang C, Page ST. Daily Oral Administration of the Novel Androgen 11β-MNTDC Markedly Suppresses Serum Gonadotropins in Healthy Men. The Journal of Clinical Endocrinology and Metabolism. March 2020, 105 (3): e835–e847. PMC 7049261 . PMID 31976519. doi:10.1210/clinem/dgaa032.
- ^ Shapiro LJ, Shapiro DB. Low Anabolic Profile in Assessing a Patient's Overall Hair Loss. 2018: 687–698. ISBN 978-4-431-56545-1. doi:10.1007/978-4-431-56547-5_72.
| 这是一篇关于类固醇的小作品。您可以通过编辑或修订扩充其内容。 |