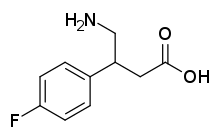
4-氨基-3-(4-氟苯基)丁酸
4-氨基-3-(4-氟苯基)丁酸或β-氨基甲基-4-氟苯丙酸(研发代号CGP-11130)是一种有机化合物,化学式为C10H12FNO2。它是GABAB受体激动剂,但从未上市。[1]它可由4-硝基-3-(4-氟苯基)丁醛经氧化至羧酸,再由硼氢化钠还原硝基,调节pH至3~4得到。[2]
 | |
| 临床资料 | |
|---|---|
| 其他名称 | CGP-11130; β-(4-Fluorophenyl)-γ-aminobutyric acid; β-(4-Fluorophenyl)-GABA; Baflofen; Fluorophenibut; F-Phenibut; Fluoribut |
| 给药途径 | By mouth |
| 识别信息 | |
| |
| CAS号 | 52237-19-1 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
| 化学式 | C10H12FNO2 |
| 摩尔质量 | 197.21 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
参考文献
编辑- ^ Bowery NG, Hill DR, Hudson AL. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br. J. Pharmacol. 1983, 78 (1): 191–206. PMC 2044790 . PMID 6297646. doi:10.1111/j.1476-5381.1983.tb09380.x.
- ^ Lieuwe Biewenga, Thangavelu Saravanan, Andreas Kunzendorf, Jan-Ytzen van der Meer, Tjaard Pijning, Pieter G. Tepper, Ronald van Merkerk, Simon J. Charnock, Andy-Mark W. H. Thunnissen, Gerrit J. Poelarends. Enantioselective Synthesis of Pharmaceutically Active γ-Aminobutyric Acids Using a Tailor-Made Artificial Michaelase in One-Pot Cascade Reactions. ACS Catalysis. 2019-02-01, 9 (2): 1503–1513 [2021-10-06]. ISSN 2155-5435. PMC 6366683 . PMID 30740262. doi:10.1021/acscatal.8b04299. (原始内容存档于2021-10-06) (英语).
- ^ RINKOS RIBOJIMO PRIEMONĖS FENIBUTUI!. ntakd.lrv.lt. [2020-01-27]. (原始内容存档于2021-10-06) (立陶宛语).
- ^ MAGYARORSZÁG HIVATALOS LAPJA (页面存档备份,存于互联网档案馆). Retrieved 2021-04-28.
| 这是一篇作用于神经系统的药品小作品。您可以通过编辑或修订扩充其内容。 |