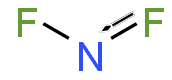
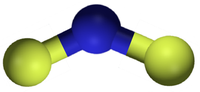
二氟化氮
二氟化氮, 又称二氟氨基自由基,是一种自由基,化学式 NF2•。这个小分子和它的二聚体四氟肼存在平衡。[2]
| 二氟化氮 | |
|---|---|
| |
| |
| 识别 | |
| CAS号 | 3744-07-8 [1] |
| PubChem | 138039 |
| ChemSpider | 121681 |
| SMILES |
|
| 性质 | |
| 化学式 | NF 2 |
| 相关物质 | |
| 相关化合物 | Nitrogen trifluoride dinitrogen tetrafluoride nitrogen monofluoride |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
N2F4 ⇌ 2 NF2•
其中,温度越高, NF2• 的含量就越多(与二氧化氮一样)[3]。
这种分子有奇数个电子,也足够稳定到可以在实验上研究。[4]
性质
编辑断裂N
2F
4的N–N键需要20.8 kcal/mol(87 kJ/mol)的能量。[5]作为比较,N
2O
4的N–N键能为14.6 kcal/mol(61 kJ/mol)、N
2O
2中为10.2 kcal/mol(43 kJ/mol),N
2H
4中则为60 kcal/mol(250 kJ/mol)。N
2F
4的标准生成焓(ΔHf)是8.227 kcal/mol(34.421 kJ/mol)。[6]
在室温下,只有0.7%的N
2F
4会离解成NF
2。到了225 °C,99%的N
2F
4都会离解成NF
2。[5]
用处
编辑二氟化氮是产生一氟化氙激发态激光的中间产物。三氟化氮是激光的制备原料,当三氟化氮被电子撞击时,会产生二氟化氮自由基和自由的氟离子:[1]
NF3 + e− → NF2 + F−
自由氟离子会和氙阳离子反应。[1]
二氟化氮可以继续反应,生成一氟化氮和氟离子:
NF2• + e− → NF + F−[1]
参考资料
编辑- ^ 1.0 1.1 1.2 1.3 Trainor, Daniel W. Electron dissociative attachment to nitrogen difluoride radicals. The Journal of Physical Chemistry. February 1989, 93 (3): 1134–1136. doi:10.1021/j100340a022.
- ^ F Fluorine: Compounds with Oxygen and Nitrogen. Gmelin Handbook of Inorganic Chemistry 4. Berlin: Springer. 1986: 162 [29 August 2015]. ISBN 978-3-662-06341-5. doi:10.1007/978-3-662-06339-2. (原始内容存档于2020-06-23). (页面存档备份,存于互联网档案馆)
- ^ Johnson, Frederic A.; Colburn, Charles B. The Tetrafluorohydrazine-Difluoroamino Radical Equilibrium. Journal of the American Chemical Society. July 1961, 83 (14): 3043–3047. doi:10.1021/ja01475a018.
- ^ Brown, R. D.; Burden, F. R.; Hart, B. T.; Williams, G. R. The electronic structure of the NF2 radical. Theoretica Chimica Acta. 1973, 28 (4): 339–353. doi:10.1007/BF00529015.
- ^ 5.0 5.1 Bohn, Robert K.; Bauer, Simon Harvey. An electron diffraction study of the structures of NF2 and N2F4. Inorganic Chemistry. February 1967, 6 (2): 304–309. doi:10.1021/ic50048a024. molecule dimensions and angles
- ^ Nitrogen difluoride NF2(g). [2022-10-27]. (原始内容存档于2020-07-10).
- ^ Brown, R.D.; Burden, F.R.; Godfrey, P.D.; Gillard, I.R. Microwave spectrum of NF2. Journal of Molecular Spectroscopy. August 1974, 52 (2): 301–321. Bibcode:1974JMoSp..52..301B. doi:10.1016/0022-2852(74)90121-0.
扩展阅读
编辑- Goodfriend, P.L.; Woods, H.P. The absorption spectrum of NF2. Journal of Molecular Spectroscopy. January 1964, 13 (1–4): 63–66. Bibcode:1964JMoSp..13...63G. doi:10.1016/0022-2852(64)90055-4.
- Jacox, Marilyn E.; Milligan, Dolphus E.; Guillory, William A.; Smith, Jerry J. Matrix-isolation study of the vacuum-ultraviolet photolysis of NF3. Journal of Molecular Spectroscopy. August 1974, 52 (2): 322–327. Bibcode:1974JMoSp..52..322J. doi:10.1016/0022-2852(74)90122-2.
- Heidner, R. F.; Helvajian, Henry; Koffend, J. Brooke. Tunable UV laser photolysis of NF2: Quantum yield for NF(a1Δ) production. The Journal of Chemical Physics. August 1987, 87 (3): 1520–1524. Bibcode:1987JChPh..87.1520H. doi:10.1063/1.453262.[失效連結]
- Papakondylis, Aristotle; Mavridis, Aristides. Electronic and geometrical structure of the NF2 radical (PDF). Chemical Physics Letters. December 1993, 216 (1–2): 167–172 [2020-06-23]. Bibcode:1993CPL...216..167P. doi:10.1016/0009-2614(93)E1254-E. (原始内容存档 (PDF)于2017-08-14). (页面存档备份,存于互联网档案馆)
- Cai, Z.-L.; Sha, G.-H.; Zhang, C.-H.; Huang, M.-B. Ab initio study of low-lying electronic states of the NF2 radical. Chemical Physics Letters. March 1991, 178 (2–3): 273–278. Bibcode:1991CPL...178..273C. doi:10.1016/0009-2614(91)87068-M.