哈米克反应
哈米克反应(英語:Hammick reaction),得名於英国化学家达尔齐尔·哈米克,指在羰基化合物存在下,α-吡啶甲酸及其类似物受热转变为2-吡啶基原醇。[1][2][3]
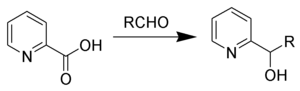
反应机理
编辑加热时,α-吡啶甲酸发生脱羧,生成两性离子(2)。该离子与强亲电试剂(如醛)作用,发生对羰基的亲核加成。加成产物发生质子转移,便得到原醇(4)。
参见
编辑参考资料
编辑- ^ Dyson, P.; Hammick, D. L. 362. Experiments on the mechanism of decarboxylation. Part I. Decomposition of quinaldinic and isoquinaldinic acids in the presence of compounds containing carbonyl groups. J. Chem. Soc. 1937: 1724. doi:10.1039/jr9370001724.
- ^ Hammick, D. L.; Dyson, P. 172. The mechanism of decarboxylation. Part II. The production of cyanide-like ions from α-picolinic, quinaldinic, and isoquinaldinic acids. J. Chem. Soc. 1939: 809. doi:10.1039/jr9390000809.
- ^ Brown, E. V.; Shambhu, M. B. Hammick reaction of methoxypyridine-2-carboxylic acids with benzaldehyde. Preparation of methoxy-2-pyridyl phenyl ketones. J. Org. Chem. 1971, 36: 2002. doi:10.1021/jo00813a034.
- ^ Sperber, N.; Papa, D.; Schwenk, E.; Sherlock, M. Pyridyl-Substituted Alkamine Ethers as Antihistaminic Agents. J. Am. Chem. Soc. 1949, 71: 887. doi:10.1021/ja01171a034.