萨伊伊波拉病毒
埃博拉病毒科
萨伊伊波拉病毒(Zaire ebolavirus,EBOV)旧称EBOV-Z或ZEBOV,是丝状病毒科伊波拉病毒属的一种病毒,为该属的六种病毒之一[1],与苏丹伊波拉病毒(SUDV)、本迪布焦伊波拉病毒(BDBV)和象牙海岸伊波拉病毒(TAFV)皆可导致伊波拉出血热。历史上多数伊波拉病毒疫情皆是此病毒株感染所致,例如2013年至2016年的西非伊波拉病毒疫情[2],共有28,646人感染,其中11,323人死亡[3]。
| 萨伊伊波拉病毒 | |
|---|---|
| |
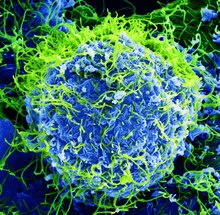
| 电子显微镜下的伊波拉病毒(绿色)与被其感染的细胞(蓝色) | |
| 病毒分类 | |
| (未分级): | 病毒 Virus |
| 域: | 核糖病毒域 Riboviria |
| 界: | 正核糖病毒界 Orthornavirae |
| 门: | 负核糖病毒门 Negarnaviricota |
| 纲: | 单荆病毒纲 Monjiviricetes |
| 目: | 单股反链病毒目 Mononegavirales |
| 科: | 丝状病毒科 Filoviridae |
| 属: | 伊波拉病毒属 Ebolavirus |
| 种: | 萨伊伊波拉病毒 Zaire ebolavirus
|
萨伊伊波拉病毒得名自其发现地萨伊共和国伊波拉河(今刚果民主共和国)[1],1976年8月该国北部亚布库(近伊波拉河)爆发疫情[4],致病病原最初被认为是马尔堡病毒的新毒株[5][6][7],1998年被正式命名为萨伊伊波拉病毒[8][9],在文献中常被简称为伊波拉病毒。此病毒的原型为Mayinga株(EBOV/May),得名自1976年疫情中逝世的护士Mayinga N'Seka[1][10][11]。
萨伊伊波拉病毒的感染发生于刚果民主共和国、加彭与刚果共和国,具2或3组重叠基因(VP35/VP40、GP/VP30与VP24/L,其中后者视VP24使用的转译终止信号而定)[1]。rVSV-ZEBOV疫苗可有效防制萨伊伊波拉病毒感染[12][13](但对苏丹伊波拉病毒防治效果不明),于2019年12月获美国食品药物管理局批准[14]。
参考文献
编辑- ^ 1.0 1.1 1.2 1.3 Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, et al. Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations. Archives of Virology. 2010, 155 (12): 2083–2103. PMC 3074192 . PMID 21046175. doi:10.1007/s00705-010-0814-x.
- ^ Na, Woonsung; Park, Nanuri; Yeom, Minju; Song, Daesub. Ebola outbreak in Western Africa 2014: what is going on with Ebola virus?. Clinical and Experimental Vaccine Research. 4 December 2016, 4 (1): 17–22. ISSN 2287-3651. PMC 4313106 . PMID 25648530. doi:10.7774/cevr.2015.4.1.17.
- ^ Ebola virus disease outbreak. World Health Organization. [4 December 2016]. (原始内容存档于2021-03-23).
- ^ Isaacson M, Sureau P, Courteille G, Pattyn, SR. Clinical Aspects of Ebola Virus Disease at the Ngaliema Hospital, Kinshasa, Zaire, 1976. European Network for Diagnostics of "Imported" Viral Diseases (ENIVD). [2014-06-24]. (原始内容存档于2014-08-04).
- ^ Pattyn S, Jacob W, van der Groen G, Piot P, Courteille G. Isolation of Marburg-like virus from a case of haemorrhagic fever in Zaire. Lancet. 1977, 309 (8011): 573–574. PMID 65663. S2CID 33060636. doi:10.1016/s0140-6736(77)92002-5.
- ^ Bowen ETW, Lloyd G, Harris WJ, Platt GS, Baskerville A, Vella EE. Viral haemorrhagic fever in southern Sudan and northern Zaire. Preliminary studies on the aetiological agent. Lancet. 1977, 309 (8011): 571–573. PMID 65662. S2CID 3092094. doi:10.1016/s0140-6736(77)92001-3.
- ^ Brown, Rob. The virus detective who discovered Ebola. BBC News. 2014-07-18 [2022-10-29]. (原始内容存档于2021-10-26).
- ^ Netesov SV, Feldmann H, Jahrling PB, Klenk HD, Sanchez A. Family Filoviridae. van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB (编). Virus Taxonomy – Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press. 2000: 539–548. ISBN 978-0123702005.
- ^ Pringle, C. R. Virus taxonomy – San Diego 1998. Archives of Virology. 1998, 143 (7): 1449–1459. PMID 9742051. S2CID 13229117. doi:10.1007/s007050050389.
- ^ Wahl-Jensen V, Kurz SK, Hazelton PR, Schnittler HJ, Stroher U, Burton DR, Feldmann H. Role of Ebola Virus Secreted Glycoproteins and Virus-Like Particles in Activation of Human Macrophages. Journal of Virology. 2005, 79 (4): 2413–2419. PMC 546544 . PMID 15681442. doi:10.1128/JVI.79.4.2413-2419.2005.
- ^ Kesel AJ, Huang Z, Murray MG, Prichard MN, Caboni L, Nevin DK, Fayne D, Lloyd DG, Detorio MA, Schinazi RF. Retinazone inhibits certain blood-borne human viruses including Ebola virus Zaire. Antiviral Chemistry & Chemotherapy. 2014, 23 (5): 197–215. PMC 7714485 . PMID 23636868. S2CID 34249020. doi:10.3851/IMP2568.
- ^ Henao-Restrepo, Ana Maria; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). The Lancet. 22 December 2016, 389 (10068): 505–518. PMC 5364328 . PMID 28017403. doi:10.1016/S0140-6736(16)32621-6.
- ^ Berlinger, Joshua. Ebola vaccine gives 100% protection, study finds. CNN. 22 December 2016 [27 December 2016]. (原始内容存档于2016-12-27).
- ^ First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response. U.S. Food and Drug Administration (FDA). 19 December 2019 [19 December 2019]. (原始内容存档于20 December 2019).