胱天蛋白酶3
胱天蛋白酶3(英語:Caspase 3)是一種在人類中由CASP3基因編碼的酶。該酶能與胱天蛋白酶8和胱天蛋白酶9產生相互作用。許多可獲得完整基因組數據的哺乳動物都已鑑定出CASP3直系同源物。[5]鳥類、蜥蜴、滑體動物和真骨類中也存在獨特的直系同源物。
胱天蛋白酶3是胱天蛋白酶(Caspase)家族的成員。[6]胱天蛋白酶的連續活化在細胞凋亡的執行階段發揮着核心作用。胱天蛋白酶以無活性的酶原形式存在,在保守的天冬氨酸殘基處經歷蛋白水解加工,產生一大一小兩個亞基,然後二聚化形成活性酶。該蛋白酶可裂解並活化胱天蛋白酶6和7,其本身則由胱天蛋白酶8、9和10加工和活化。該蛋白酶是參與裂解前類澱粉蛋白質的主要胱天蛋白酶,而前類澱粉蛋白質與阿茲海默症中的神經元死亡有關。[7]該基因的選擇性剪接會產生編碼相同蛋白質的兩個轉錄變體。[8]
 |
 |
胱天蛋白酶3具有許多目前已知的胱天蛋白酶共有的典型特徵。例如,其活性位點包含半胱氨酸殘基(Cys-163)和組氨酸殘基(His-121),當它位於特定的4個氨基酸序列中時,它能夠穩定蛋白質序列肽鍵裂解到天冬氨酸的羧基末端側。[10][11]這種特異性使得胱天蛋白酶具有有極高的選擇性,對天冬氨酸的偏好是穀氨酸的2萬倍。[12]胱天蛋白酶在細胞中的一個關鍵特徵是它們以未活化的前酶形式存在,稱為胱天蛋白酶原,直到生化變化引起它們的活化為止。每個胱天蛋白酶原都有一個約20kDa的N端大亞基,後面跟着一個約10kDa的小亞基,分別稱為p20和p10。[13]
基質特異性
編輯正常情況下,胱天蛋白酶識別其基質上的四肽序列並水解天冬氨酸殘基後的肽鍵。胱天蛋白酶3和7通過識別四肽基序Asp-x-x-Asp共享類似的基質特異性。[14]C端天冬氨酸是絕對必需的,而其他三個位置的變化是可以容忍的。[15]胱天蛋白酶基質特異性已廣泛應用於基於胱天蛋白酶的抑制劑和藥物設計。[16]
結構
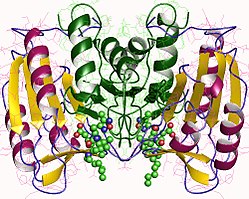
編輯胱天蛋白酶3(也稱為CPP32、Yama或apopain)[17][18][19]是由一個32kDa的酶原形成,該酶原被切割成17kDa和12kDa亞基。當胱天蛋白酶原在特定殘基處裂解時,活性異四聚體就能通過疏水相互作用形成,導致來自p17的四個反平行β摺疊和來自p12的兩個反平行β摺疊結合在一起形成異二聚體,該異二聚體又與另一個異二聚體相互作用形成完整的由α螺旋包圍的12鏈β摺疊結構,這是胱天蛋白酶特有的。[13][20]當異二聚體頭尾相連時,分子兩端各有一個由兩個參與亞基的殘基形成的活性位點,儘管必要的Cys-163和His-121殘基位於p17(較大的)亞基上。[20]
機制
編輯胱天蛋白酶3的催化位點涉及Cys-163的硫醇基團和His-121的咪唑環。His-121穩定關鍵天冬氨酸殘基的羰基,而Cys-163則攻擊最終裂解肽鍵。Cys-163和Gly-238還可以通過氫鍵穩定基質-酶複合物的四面體過渡態。[20]在體外,已發現胱天蛋白酶3更喜歡肽序列 DEVDG(Asp-Glu-Val-Asp-Gly),其切割發生在第二個天冬氨酸殘基的羧基側(D和G之間)。[12][20][21]胱天蛋白酶3在較寬的pH範圍內具有活性,該範圍比許多其他執行型胱天蛋白酶稍高(鹼性更強)。這一廣泛的範圍表明胱天蛋白酶3在正常和凋亡細胞條件下都可以完全活躍。[22]
活化
編輯胱天蛋白酶3在凋亡細胞中通過外在(死亡配體)和內在(線粒體)途徑被活化。[13][23]胱天蛋白酶3的酶原特徵是必要的,因為如果不受調節,胱天蛋白酶的活性會不加區別地殺死細胞。[24]作為執行型胱天蛋白酶,胱天蛋白酶3酶原實際上沒有活性,直到凋亡信號事件發生後被啟動型胱天蛋白酶切割。[25]此類信號事件之一是將顆粒酶B引入殺傷性T細胞針對凋亡的細胞中,該顆粒酶B可以活化啟動型胱天蛋白酶。[26][27]這種外在活化隨後觸發細胞凋亡途徑的標誌性胱天蛋白酶級聯特徵,其中胱天蛋白酶3發揮主導作用。[11]在內在活化過程中,來自線粒體的細胞色素c與胱天蛋白酶9、凋亡活化因子1(Apaf-1)和ATP結合作用來處理胱天蛋白酶3酶原。[21][27][28]這些分子足以在體外活化胱天蛋白酶3,但體內還需要其他調節蛋白。[28]山竹(Garcinia mangostana)提取物已被證明可以抑制β澱粉樣蛋白處理的人類神經元細胞中胱天蛋白酶3的活化。[29]
抑制
編輯抑制胱天蛋白酶的一種方法是通過IAP(凋亡抑制劑)蛋白家族,其中包括c-IAP1、c-IAP2、XIAP和ML-IAP。[20]XIAP結合併抑制啟動型胱天蛋白酶9,後者直接參與執行型胱天蛋白酶3的活化。[28]然而,在胱天蛋白酶級聯過程中,胱天蛋白酶3通過在特定位點切割胱天蛋白酶9來抑制XIAP的活性,從而阻止XIAP結合來抑制胱天蛋白酶9的活性。[30]
相互作用
編輯胱天蛋白酶3已被證明可以與以下物質相互作用:
生物學功能
編輯人們發現胱天蛋白酶3對於正常的大腦發育是必需的,它在細胞凋亡中也發揮着典型作用,負責染色質濃縮和DNA碎裂。[21]血液中胱天蛋白酶3片段(p17)水平升高是近期心肌梗塞的徵兆。[52]現在的研究表明,胱天蛋白酶3可能在胚胎和造血幹細胞分化中發揮作用。[53]
參見
編輯- 蛋白酶解圖譜
- 胱天蛋白酶
- 半胱天冬酶原活化物1(PAC-1)
參考文獻
編輯- ^ 對Caspase 3起作用的藥物;在維基數據上查看/編輯參考.
- ^ 2.0 2.1 2.2 GRCm38: Ensembl release 89: ENSMUSG00000031628 - Ensembl, May 2017
- ^ Human PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Mouse PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ OrthoMaM phylogenetic marker: CASP3 coding sequence. [2009-12-20]. (原始內容存檔於2016-03-03).
- ^ Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. October 1996, 87 (2): 171. PMID 8861900. S2CID 5345060. doi:10.1016/S0092-8674(00)81334-3 .
- ^ Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. April 1999, 97 (3): 395–406. PMID 10319819. S2CID 17524567. doi:10.1016/s0092-8674(00)80748-5 .
- ^ Entrez Gene: CASP3 caspase 3, apoptosis-related cysteine peptidase.
- ^ Harrington HA, Ho KL, Ghosh S, Tung KC. Construction and analysis of a modular model of caspase activation in apoptosis. Theoretical Biology & Medical Modelling. 2008, 5 (1): 26. PMC 2672941 . PMID 19077196. doi:10.1186/1742-4682-5-26 .
- ^ Wyllie AH. Apoptosis: an overview. British Medical Bulletin. 1997, 53 (3): 451–65. PMID 9374030. doi:10.1093/oxfordjournals.bmb.a011623 .
- ^ 11.0 11.1 Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. The Journal of Biological Chemistry. July 1997, 272 (30): 18530–3. PMID 9228015. doi:10.1074/jbc.272.30.18530 .
- ^ 12.0 12.1 Stennicke HR, Renatus M, Meldal M, Salvesen GS. Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8. The Biochemical Journal. September 2000, 350 (2): 563–8. PMC 1221285 . PMID 10947972. doi:10.1042/0264-6021:3500563.
- ^ 13.0 13.1 13.2 Salvesen GS. Caspases: opening the boxes and interpreting the arrows. Cell Death and Differentiation. January 2002, 9 (1): 3–5. PMID 11803369. S2CID 31274387. doi:10.1038/sj.cdd.4400963.
- ^ Agniswamy J, Fang B, Weber IT. Plasticity of S2-S4 specificity pockets of executioner caspase-7 revealed by structural and kinetic analysis. The FEBS Journal. September 2007, 274 (18): 4752–65. PMID 17697120. S2CID 1860924. doi:10.1111/j.1742-4658.2007.05994.x.
- ^ Fang B, Boross PI, Tozser J, Weber IT. Structural and kinetic analysis of caspase-3 reveals role for s5 binding site in substrate recognition. Journal of Molecular Biology. July 2006, 360 (3): 654–66. PMID 16781734. doi:10.1016/j.jmb.2006.05.041.
- ^ Weber IT, Fang B, Agniswamy J. Caspases: structure-guided design of drugs to control cell death. Mini Reviews in Medicinal Chemistry. October 2008, 8 (11): 1154–62. PMID 18855730. doi:10.2174/138955708785909899.
- ^ Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. The Journal of Biological Chemistry. December 1994, 269 (49): 30761–4. PMID 7983002. doi:10.1016/S0021-9258(18)47344-9 .
- ^ Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. June 1995, 81 (5): 801–9. PMID 7774019. S2CID 18866447. doi:10.1016/0092-8674(95)90541-3 .
- ^ Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. July 1995, 376 (6535): 37–43. Bibcode:1995Natur.376...37N. PMID 7596430. S2CID 4240789. doi:10.1038/376037a0.
- ^ 20.0 20.1 20.2 20.3 20.4 Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. The Journal of Clinical Investigation. October 2005, 115 (10): 2665–72. PMC 1236692 . PMID 16200200. doi:10.1172/JCI26252.
- ^ 21.0 21.1 21.2 Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death and Differentiation. February 1999, 6 (2): 99–104. PMID 10200555. doi:10.1038/sj.cdd.4400476 .
- ^ Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. The Journal of Biological Chemistry. October 1997, 272 (41): 25719–23. PMID 9325297. doi:10.1074/jbc.272.41.25719 .
- ^ Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M. Apoptosis and cancer: mutations within caspase genes. Journal of Medical Genetics. August 2009, 46 (8): 497–510. PMID 19505876. doi:10.1136/jmg.2009.066944 .
- ^ Boatright KM, Salvesen GS. Mechanisms of caspase activation. Current Opinion in Cell Biology. December 2003, 15 (6): 725–31. PMID 14644197. doi:10.1016/j.ceb.2003.10.009.
- ^ Walters J, Pop C, Scott FL, Drag M, Swartz P, Mattos C, Salvesen GS, Clark AC. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. The Biochemical Journal. December 2009, 424 (3): 335–45. PMC 2805924 . PMID 19788411. doi:10.1042/BJ20090825.
- ^ Gallaher BW, Hille R, Raile K, Kiess W. Apoptosis: live or die--hard work either way!. Hormone and Metabolic Research. September 2001, 33 (9): 511–9. PMID 11561209. S2CID 36623826. doi:10.1055/s-2001-17213.
- ^ 27.0 27.1 Katunuma N, Matsui A, Le QT, Utsumi K, Salvesen G, Ohashi A. Novel procaspase-3 activating cascade mediated by lysoapoptases and its biological significances in apoptosis. Advances in Enzyme Regulation. 2001, 41 (1): 237–50. PMID 11384748. doi:10.1016/S0065-2571(00)00018-2.
- ^ 28.0 28.1 28.2 Li P, Nijhawan D, Wang X. Mitochondrial activation of apoptosis. Cell. January 2004, 116 (2 Suppl): S57–9, 2 p following S59. PMID 15055583. S2CID 5180966. doi:10.1016/S0092-8674(04)00031-5 .
- ^ Moongkarndi P, Srisawat C, Saetun P, Jantaravinid J, Peerapittayamongkol C, Soi-ampornkul R, Junnu S, Sinchaikul S, Chen ST, Charoensilp P, Thongboonkerd V, Neungton N. Protective effect of mangosteen extract against beta-amyloid-induced cytotoxicity, oxidative stress and altered proteome in SK-N-SH cells (PDF). Journal of Proteome Research. May 2010, 9 (5): 2076–86 [2024-01-25]. PMID 20232907. doi:10.1021/pr100049v. (原始內容存檔 (PDF)於2023-08-04).
- ^ Denault JB, Eckelman BP, Shin H, Pop C, Salvesen GS. Caspase 3 attenuates XIAP (X-linked inhibitor of apoptosis protein)-mediated inhibition of caspase 9. The Biochemical Journal. July 2007, 405 (1): 11–9. PMC 1925235 . PMID 17437405. doi:10.1042/BJ20070288.
- ^ Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. The Journal of Biological Chemistry. April 2002, 277 (16): 13430–7. PMID 11832478. doi:10.1074/jbc.M108029200 .
- ^ Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proceedings of the National Academy of Sciences of the United States of America. December 1996, 93 (25): 14486–91. Bibcode:1996PNAS...9314486S. PMC 26159 . PMID 8962078. doi:10.1073/pnas.93.25.14486 .
- ^ Selvakumar, P.; Sharma, RK. Role of calpain and caspase system in the regulation of N-myristoyltransferase in human colon cancer (Review).. Int J Mol Med. May 2007, 19 (5): 823–7. PMID 17390089. doi:10.3892/ijmm.19.5.823 .
- ^ Shu HB, Halpin DR, Goeddel DV. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity. June 1997, 6 (6): 751–63. PMID 9208847. doi:10.1016/S1074-7613(00)80450-1 .
- ^ Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM, Hood L. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proceedings of the National Academy of Sciences of the United States of America. October 1997, 94 (21): 11333–8. Bibcode:1997PNAS...9411333H. PMC 23459 . PMID 9326610. doi:10.1073/pnas.94.21.11333 .
- ^ Forcet C, Ye X, Granger L, Corset V, Shin H, Bredesen DE, Mehlen P. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proceedings of the National Academy of Sciences of the United States of America. March 2001, 98 (6): 3416–21. Bibcode:2001PNAS...98.3416F. PMC 30668 . PMID 11248093. doi:10.1073/pnas.051378298 .
- ^ Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. The EMBO Journal. April 1999, 18 (8): 2040–8. PMC 1171288 . PMID 10205158. doi:10.1093/emboj/18.8.2040.
- ^ Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson DW. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. The EMBO Journal. April 1999, 18 (8): 2049–56. PMC 1171289 . PMID 10205159. doi:10.1093/emboj/18.8.2049.
- ^ Ruzzene M, Penzo D, Pinna LA. Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. The Biochemical Journal. May 2002, 364 (Pt 1): 41–7. PMC 1222543 . PMID 11988074. doi:10.1042/bj3640041.
- ^ Chen YR, Kori R, John B, Tan TH. Caspase-mediated cleavage of actin-binding and SH3-domain-containing proteins cortactin, HS1, and HIP-55 during apoptosis. Biochemical and Biophysical Research Communications. November 2001, 288 (4): 981–9. PMID 11689006. doi:10.1006/bbrc.2001.5862.
- ^ Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Research. December 1998, 58 (23): 5315–20. PMID 9850056.
- ^ Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. January 2001, 40 (4): 1117–23. PMID 11170436. doi:10.1021/bi001603q.
- ^ Lee ZH, Lee SE, Kwack K, Yeo W, Lee TH, Bae SS, Suh PG, Kim HH. Caspase-mediated cleavage of TRAF3 in FasL-stimulated Jurkat-T cells. Journal of Leukocyte Biology. March 2001, 69 (3): 490–6. PMID 11261798. S2CID 34256107. doi:10.1189/jlb.69.3.490.
- ^ Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC. TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-alpha-induced apoptosis. The Journal of Biological Chemistry. March 2001, 276 (11): 8087–93. PMID 11098060. doi:10.1074/jbc.M009450200 .
- ^ Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proceedings of the National Academy of Sciences of the United States of America. July 2001, 98 (15): 8662–7. Bibcode:2001PNAS...98.8662S. PMC 37492 . PMID 11447297. doi:10.1073/pnas.161506698 .
- ^ Silke J, Hawkins CJ, Ekert PG, Chew J, Day CL, Pakusch M, Verhagen AM, Vaux DL. The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites. The Journal of Cell Biology. April 2002, 157 (1): 115–24. PMC 2173256 . PMID 11927604. doi:10.1083/jcb.200108085.
- ^ Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. March 2001, 104 (5): 791–800. PMID 11257232. S2CID 17915093. doi:10.1016/S0092-8674(01)00274-4 .
- ^ Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. The EMBO Journal. December 1997, 16 (23): 6914–25. PMC 1170295 . PMID 9384571. doi:10.1093/emboj/16.23.6914.
- ^ Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. July 1997, 388 (6639): 300–4. Bibcode:1997Natur.388..300D. PMID 9230442. S2CID 4395885. doi:10.1038/40901 .
- ^ Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R. X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. The Journal of Biological Chemistry. July 2001, 276 (29): 27058–63. PMID 11359776. doi:10.1074/jbc.M102415200 .
- ^ Ohtsubo T, Kamada S, Mikami T, Murakami H, Tsujimoto Y. Identification of NRF2, a member of the NF-E2 family of transcription factors, as a substrate for caspase-3(-like) proteases. Cell Death and Differentiation. September 1999, 6 (9): 865–72. PMID 10510468. doi:10.1038/sj.cdd.4400566 .
- ^ Agosto M, Azrin M, Singh K, Jaffe AS, Liang BT. Serum caspase-3 p17 fragment is elevated in patients with ST-segment elevation myocardial infarction: a novel observation. Journal of the American College of Cardiology. January 2011, 57 (2): 220–1. PMID 21211695. doi:10.1016/j.jacc.2010.08.628 .
- ^ Abdul-Ghani M, Megeney LA. Rehabilitation of a contract killer: caspase-3 directs stem cell differentiation. Cell Stem Cell. June 2008, 2 (6): 515–6. PMID 18522841. doi:10.1016/j.stem.2008.05.013 .
拓展閱讀
編輯- Cohen GM. Caspases: the executioners of apoptosis. The Biochemical Journal. August 1997, 326 (Pt 1): 1–16. PMC 1218630 . PMID 9337844. doi:10.1042/bj3260001.
- Roig J, Traugh JA. Cytostatic p21 G protein-activated protein kinase gamma-PAK. Vitamins & Hormones 62. 2001: 167–98. ISBN 9780127098623. PMID 11345898. doi:10.1016/S0083-6729(01)62004-1.
- Zhao LJ, Zhu H. Structure and function of HIV-1 auxiliary regulatory protein Vpr: novel clues to drug design. Current Drug Targets. Immune, Endocrine and Metabolic Disorders. December 2004, 4 (4): 265–75. PMID 15578977. doi:10.2174/1568008043339668.
- Le Rouzic E, Benichou S. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology. 2006, 2 (1): 11. PMC 554975 . PMID 15725353. doi:10.1186/1742-4690-2-11 .
- Sykes MC, Mowbray AL, Jo H. Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death. Circulation Research. February 2007, 100 (2): 152–4. PMID 17272816. S2CID 12684325. doi:10.1161/01.RES.0000258171.08020.72.