硼烯
硼烯是硼原子的单分子层结晶,也就是说它是硼的二维同素异形体。硼烯最早在1990年代中期被理论预言,[1]2015年在实验中不同结构的硼烯被证实存在。[2][3]
性质
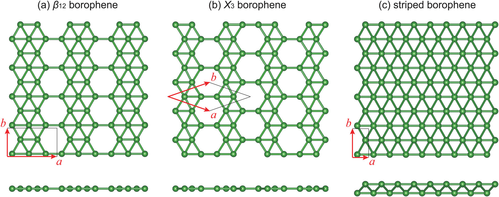
编辑在超高真空条件下的洁净金属表面上,不同种类的硼烯晶体和金属可以以原子尺度的薄片形式制备。[2][3]如图1所示,它们的原子结构由三角和六边形图样混合而成。其原子结构是平面内双中心和多中心的成键的结果,这对于硼这样的缺电子元素来说非常典型。[4]
硼烯在面内有具有弹性和理想的强度。在某些情况下它可以比石墨烯更坚固且更柔韧。[5]例如,硼纳米管比其他所有已知的碳和非碳的纳米结构都有更高的二维杨氏模量。[6]由于硼烯面内多中心键合的流变性,面内的拉伸载荷下会使得硼烷发生独特的结构相变。[7]理论上硼烯有着更高的比热容,电导率和离子传输特性,它有作为电池阳极材料的潜力。氢很容易吸附到硼苯上,使它具有作为储氢材料的潜力——可以储存其重量15%以上的氢。硼烯可以催化氢分子分解成氢离子,并催化分解水。
历史
编辑36簇可能是最小的硼烯;前视图和侧视图
I. Boustani和A. Quandt的计算研究表明,小的硼团簇不像硼烷那样是正二十面体,相反它们被发现是准平面的(见图2)。[1]这导致了所谓的Aufbau原理被发现[8] ,该原理预测了硼烯、硼富勒烯(硼球烯)[9]和硼纳米管存在的可能性。[10][11][12]
进一步的研究表明,扩展的三角形硼烯(图1(c))是金属性的,并有着非平面的弯曲的几何形状。[13][14]进一步的从稳定B80硼富勒烯开始的计算[15]表明,有着蜂窝结构并部分由六边形孔填充的扩展的片状硼烯是稳定的。[16][17]这些硼烯的结构被预言是金属性的。所谓的γ片(β12硼烯或 υ1/6 片)示于图1(a)。
硼团簇的平面性首先由王来生的研究小组通过实验证实。[18]晚些时候他们证明了B
36结构(见图2)是具有六重对称性和完美六边形空缺的最小硼团簇,它可能可以作为扩展二维硼烯的基础。[19]
在硅烯被制备后,多个研究小组预测,硼烯有可能能在金属表面上被制取。[20][21][22]特别地,硼烯的晶格结构取决于金属表面,显示出不同于自由状态下的晶格结构。[23]
在2015年,两个研究小组成功地在超高真空条件下在银(111)表面合成了不同的硼烯的相。[2][3]在三个制取成功的硼烯的相中(见图1),υ1/6 片或 β12在早先的理论中被证明是银(111)面上的基态,[23]而χ3硼烯是Xiao Chen Zeng团队在2012年预测的。[24]目前为止硼烯仅存在于底物上,对把它们转移到与设备兼容的衬底上的方法的研究是有必要的,但也是一个挑战。[25]
在理论计算的支持下,原子尺度的表征揭示的结构让人想起混合着的包含三角和六方结构的硼团簇,正如先前的理论预言的那样,如图1所示。扫描隧道显微镜证实硼烯是金属性的。这与硼的同素异形体不同——后者是半导体并具有基于B12二十面体的原子结构。[ 需要引用 ]
参见
编辑参考文献
编辑- ^ 1.0 1.1 Boustani, Ihsan. New quasi-planar surfaces of bare boron. Surface Science. January 1997, 370 (2–3): 355–363. Bibcode:1997SurSc.370..355B. doi:10.1016/S0039-6028(96)00969-7.
- ^ 2.0 2.1 2.2 Mannix, A. J.; Zhou, X.-F.; Kiraly, B.; Wood, J. D.; Alducin, D.; Myers, B. D.; Liu, X.; Fisher, B. L.; Santiago, U. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science. 17 December 2015, 350 (6267): 1513–1516. Bibcode:2015Sci...350.1513M. PMC 4922135 . PMID 26680195. doi:10.1126/science.aad1080.
- ^ 3.0 3.1 3.2 Feng, Baojie; Zhang, Jin; Zhong, Qing; Li, Wenbin; Li, Shuai; Li, Hui; Cheng, Peng; Meng, Sheng; Chen, Lan. Experimental realization of two-dimensional boron sheets. Nature Chemistry. 28 March 2016, 8 (6): 563–568. Bibcode:2016NatCh...8..563F. PMID 27219700. arXiv:1512.05029 . doi:10.1038/nchem.2491.
- ^ Pauling, Linus. The nature of the chemical bond 3rd. Cornell University Press. 1960. ISBN 0-8014-0333-2.
- ^ arXiv, Emerging Technology from the. Sorry, graphene—borophene is the new wonder material that's got everyone excited. MIT Technology Review. [2019-08-02]. (原始内容存档于2019-10-14) (美国英语).
- ^ Kochaev, A. Elastic properties of noncarbon nanotubes as compared to carbon nanotubes. Physical Review B. 11 October 2017, 96 (15): 155428. doi:10.1103/PhysRevB.96.155428.
- ^ Zhang, Z.; Yang, Yang.; Penev, E.S.; Yakobson, B.I. Elasticity, Flexibility, and Ideal Strength of Borophenes. Advanced Functional Materials. 11 January 2017, 27 (9): 1605059. arXiv:1609.07533 . doi:10.1002/adfm.201605059.
- ^ Boustani, Ihsan. Systematic ab initio investigation of bare boron clusters: Determination of the geometry and electronic structures of Bn (n=2-14). Physical Review B. 15 June 1997, 55 (24): 16426–16438. doi:10.1103/PhysRevB.55.16426.
- ^ Boustani, Ihsan. New Convex and Spherical Structures of Bare Boron Clusters. Journal of Solid State Chemistry. October 1997, 133 (1): 182–189. doi:10.1006/jssc.1997.7424.
- ^ Boustani, I; Quandt, A. Nanotubules of bare boron clusters: Ab initio and density functional study. Europhysics Letters (EPL). 1 September 1997, 39 (5): 527–532. doi:10.1209/epl/i1997-00388-9.
- ^ Gindulytė, Asta; Lipscomb, William N.; Massa, Lou. Proposed Boron Nanotubes. Inorganic Chemistry. December 1998, 37 (25): 6544–6545. PMID 11670779. doi:10.1021/ic980559o.
- ^ Quandt, Alexander; Boustani, Ihsan. Boron Nanotubes. ChemPhysChem. 14 October 2005, 6 (10): 2001–2008. PMID 16208735. doi:10.1002/cphc.200500205.
- ^ Boustani, Ihsan; Quandt, Alexander; Hernández, Eduardo; Rubio, Angel. New boron based nanostructured materials. The Journal of Chemical Physics. 8 February 1999, 110 (6): 3176–3185. doi:10.1063/1.477976.
- ^ Kunstmann, Jens; Quandt, Alexander. Broad boron sheets and boron nanotubes: An ab initio study of structural, electronic, and mechanical properties. Physical Review B. 12 July 2006, 74 (3): 035413. arXiv:cond-mat/0509455 . doi:10.1103/PhysRevB.74.035413.
- ^ Gonzalez Szwacki, Nevill; Sadrzadeh, Arta; Yakobson, Boris I. B80 Fullerene: An Ab Initio Prediction of Geometry, Stability, and Electronic Structure. Physical Review Letters. 20 April 2007, 98 (16): 166804. Bibcode:2007PhRvL..98p6804G. PMID 17501448. doi:10.1103/PhysRevLett.98.166804.
- ^ Tang, Hui & Ismail-Beigi, Sohrab. Novel Precursors for Boron Nanotubes: The Competition of Two-Center and Three-Center Bonding in Boron Sheets. Physical Review Letters. 2007, 99 (11): 115501. Bibcode:2007PhRvL..99k5501T. PMID 17930448. arXiv:0710.0593 . doi:10.1103/PhysRevLett.99.115501.
- ^ Özdoğan, C.; Mukhopadhyay, S.; Hayami, W.; Güvenç, Z. B.; Pandey, R.; Boustani, I. The Unusually Stable B100 Fullerene, Structural Transitions in Boron Nanostructures, and a Comparative Study of α- and γ-Boron and Sheets. The Journal of Physical Chemistry C. 18 March 2010, 114 (10): 4362–4375. doi:10.1021/jp911641u.
- ^ Zhai, Hua-Jin; Kiran, Boggavarapu; Li, Jun; Wang, Lai-Sheng. Hydrocarbon analogues of boron clusters — planarity, aromaticity and antiaromaticity. Nature Materials. 9 November 2003, 2 (12): 827–833. PMID 14608377. doi:10.1038/nmat1012.
- ^ Piazza, Z. A.; Hu, H. S.; Li, W. L.; Zhao, Y. F.; Li, J.; Wang, L. S. Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nature Communications. 2014, 5: 3113. Bibcode:2014NatCo...5.3113P. PMID 24445427. doi:10.1038/ncomms4113.
- ^ Zhang, L. Z.; Yan, Q. B.; Du, S. X.; Su, G.; Gao, H.-J. Boron Sheet Adsorbed on Metal Surfaces: Structures and Electronic Properties. The Journal of Physical Chemistry C. 15 August 2012, 116 (34): 18202–18206. doi:10.1021/jp303616d.
- ^ Liu, Yuanyue; Penev, Evgeni S.; Yakobson, Boris I. Probing the Synthesis of Two-Dimensional Boron by First-Principles Computations. Angewandte Chemie International Edition. 11 March 2013, 52 (11): 3156–3159. PMID 23355180. arXiv:1312.0656 . doi:10.1002/anie.201207972.
- ^ Liu, Hongsheng; Gao, Junfeng; Zhao, Jijun. From Boron Cluster to Two-Dimensional Boron Sheet on Cu(111) Surface: Growth Mechanism and Hole Formation. Scientific Reports. 18 November 2013, 3 (1): 3238. PMC 3831238 . PMID 24241341. doi:10.1038/srep03238.
- ^ 23.0 23.1 Zhang, Z.; Yang, Y.; Gao, G.; Yakobson, B.I. Two-Dimensional Boron Monolayers Mediated by Metal Substrates. Angewandte Chemie International Edition. 2 September 2015, 54 (44): 13022–13026. PMID 26331848. doi:10.1002/anie.201505425.
- ^ Wu, Xiaojun; Dai, Jun; Zhao, Yu; Zhu, Zhiwen; Yang, Jinlong; Zeng, Xiao Cheng. Two-Dimensional Boron Monolayer Sheets. ACS Nano. 20 July 2012, 6 (8): 7443–7453. PMID 22816319. doi:10.1021/nn302696v.
- ^ Zhang, Z.; Penev, E.S.; Yakobson, B.I. Two-dimensional boron: structures, properties and applications. Chemical Society Reviews. 31 October 2017, 46 (22): 6746–6763. PMID 29085946. doi:10.1039/c7cs00261k.