硫代乙酰丙酮
硫代乙酰丙酮是一种有机化合物,化学式为C5H8OS。它可由乙酰丙酮和硫化氢在乙腈中反应制得,[1]或由丙酮、硫代乙酸-O-乙酯和叔丁基锂在乙醚中反应得到。[2]也有文献报道了[2-(二甲基氯硅基)-1-乙基-1-丙烯-1-基]乙基氯化硼和硫化物反应,得到4,5-二乙基-2,2,3-三甲基-1-硫杂-2-硅杂-5-硼杂环戊-3-烯为硫化试剂,将乙酰丙酮硫化的反应。[3]
| 硫代乙酰丙酮 | |
|---|---|
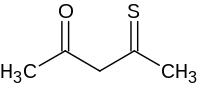
| |
| 别名 | 2-硫代-2,4-戊二酮 |
| 识别 | |
| CAS号 | 14660-20-9 |
| 性质 | |
| 化学式 | C5H8OS |
| 摩尔质量 | 116.18 g·mol−1 |
| 沸点 | 57 °C(11 torr) |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
反应
编辑硫代乙酰丙酮和三乙基氯化硅(或三乙基溴化锗)在三乙胺存在下反应,可以得到4-三乙基硅硫基-3-戊烯-2-酮(或4-三乙基锗硫基-3-戊烯-2-酮)。[4]它和乙酰丙酮一样,可以和金属形成配位化合物。[5][6]
参考文献
编辑- ^ Duus, Fritz; Anthonsen, Joergen W. β-Thioxo ketones. I. Preparation and structure of thioacetylacetone. Acta Chemica Scandinavica, Series B: Organic Chemistry and Biochemistry, 1977. B31 (1): 40-46.
- ^ Fritz Duus. Synthesis of ß-Thioxoketones: by t -Butyllithium-Promoted Claisen Condensation Reaction of Ketones with Thionoesters. Synthesis. 1985, 1985 (6/7): 672–674 [2022-05-31]. ISSN 0039-7881. doi:10.1055/s-1985-31304. (原始内容存档于2018-06-03) (英语).
- ^ Koester, Roland; Seidel, Guenter; Boese, Roland; Wrackmeyer, Bernd. Boron compounds. 80. 2,5-Dihydro-1,2,5-thiasilaboroles. Preparation and complexations. Chemische Berichte, 1988. 121 (4): 709-721. ISSN 0009-2940.
- ^ Vyazankina, O. A.; Vyazankin, N. S.; Kalikhman, I. D.; Brodskaya, E. I.; Belousova, L. I. Synthesis of silicon- and germanium-containing derivatives of thioacetylacetone. Zhurnal Obshchei Khimii, 1985. 55 (5): 1207-1208. ISSN 0044-460X.
- ^ L.J. Botha, S.S. Basson, J.G. Leipoldt. The crystal structure of thioacetylacetonatocarbonyltriphenylphosphinerhodium(I). Inorganica Chimica Acta. 1987-01, 126 (1): 25–28 [2022-05-31]. doi:10.1016/S0020-1693(00)81234-7. (原始内容存档于2018-06-27) (英语).
- ^ Walter Purcell, Jeanet Conradie, Sumit Kumar, Johan A. Venter. Characterisation and mechanistic study of the oxidative addition reactions of [Ir(cod)(sacac)]. Journal of Organometallic Chemistry. 2016-01, 801: 80–86 [2022-05-31]. doi:10.1016/j.jorganchem.2015.10.012. (原始内容存档于2018-06-14) (英语).