吡啶-2-甲酸鉻
化合物
吡啶-2-甲酸鉻(簡寫:CrPic3)是一種化合物,用作營養補充劑以治療2型糖尿病,以及促進減肥。[1] 它是紅粉色的化合物,最初於1917年報道。[2][3]它難溶於水,在中性pH下的水中,溶解度為600 µmol/L。[4]它和其它三價鉻化合物類似,化學性質上具有惰性,即在一般條件下穩定,它僅在較高溫度下分解。[5]在較低的pH下,該配合物會游離出吡啶-2-甲酸和Cr3+離子。[4]
| 吡啶-2-甲酸鉻 | |||
|---|---|---|---|
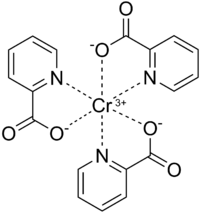
| |||
| |||

| |||
| IUPAC名 Tris(picolinate)chromium(III) | |||
| 別名 | 2-吡啶甲酸鉻 2-吡啶羧酸鉻 吡啶-2-羧酸鉻 | ||
| 識別 | |||
| CAS號 | 14639-25-9 | ||
| PubChem | 151932 | ||
| ChemSpider | 133913 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | CBDQOLKNTOMMTL-DFZHHIFOAM | ||
| ChEBI | 50369 | ||
| 性質 | |||
| 化學式 | Cr(C6H4NO2)3 | ||
| 莫耳質量 | 418.33 g/mol g·mol⁻¹ | ||
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |||
參考文獻
編輯- ^ Preuss, H. G.; Echard, B.; Perricone, N. V.; Bagchi, D.; Yasmin, T.; Stohs, S. J. Comparing metabolic effects of six different commercial trivalent chromium compounds. Journal of Inorganic Biochemistry. 2008, 102 (11): 1986–1990. PMID 18774175. doi:10.1016/j.jinorgbio.2008.07.012.
- ^ Vincent, John (2010). "Chromium: celebrating 50 years as an essential element?". Dalton Transactions. Royal Society of Chemistry. 39: 3787–3794. doi:10.1039/B920480F. PMID 20372701. Retrieved 20 March 2015.
- ^ Vincent, John. The Bioinorganic Chemistry of Chromium (III). Polyhedron (Elsevier B.V). 2001, 20: 1–26 [20 March 2015]. doi:10.1016/S0277-5387(00)00624-0. (原始內容存檔於2015-09-24).
- ^ 4.0 4.1 Feng, Weiyue (2007). "Chapter 6—The Transport of chromium (III) in the body: Implications for Function". In Vincent, John. The Nutritional Biochemistry of Chromium (III) (PDF). Amsterdam: Elsevier B.V. pp. 121–137. ISBN 978-0-444-53071-4. Retrieved 20 March 2015.
- ^ Abou–Gamra, Zeinab; Abdel–Messih, Michel. Correlation of thermal and spectral properties of chromium(III) picolinate complex and kinetic study of its thermal degradation. Journal of Thermal Analysis and Calorimetry (Springer Netherlands). 2014, 117 (2): 993–1000 [1 April 2015]. doi:10.1007/s10973-014-3768-5. (原始內容存檔於2019-07-30).