2-氰基呋喃
化合物
| 2-氰基呋喃 | |
|---|---|
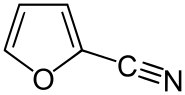
| |
| 別名 | 呋喃-2-甲腈; 2-呋喃甲腈; 2-呋喃基氰 |
| 識別 | |
| CAS號 | 617-90-3 |
| PubChem | 69245 |
| ChemSpider | 62458 |
| SMILES |
|
| InChI |
|
| InChIKey | YXDXXGXWFJCXEB-UHFFFAOYAE |
| 性質 | |
| 化學式 | C5H3NO |
| 摩爾質量 | 93.08 g·mol−1 |
| 外觀 | 無色(不純時呈黃色) |
| 密度 | 20 °C時為1.0650 g/cm-3[1] |
| 沸點 | 147 °C(420 K)[2] |
| 危險性 | |
| 閃點 | 95 °F(308 K) |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
合成方式
編輯工業上合成2-氰基呋喃主要透過在440-480℃,鉬酸鉍催化劑存在下於氣相環境中,將糠醛與氨混合行氨氧化反應。 [3]
實驗室中也有其他合成2-氰基呋喃的方法;例如,使用高價碘試劑[4]或N-溴代丁二醯亞胺作氧化劑,加入糠醛與氨鹽混合氧化脫水得到2-氰基呋喃、[5]將糠醛醛肟與亞硫醯氯-苯並三唑、[6]三苯基膦-碘試劑反應[7]或在二甲基亞碸(DMSO)中[8]加熱脫水,以及將糠醯胺透過急驟真空熱解脫水皆可獲取2-氰基呋喃。[9]
應用
編輯2-氰基呋喃目前沒有大幅度的應用,但它被用作藥物和精細化工合成的中間體。2-氰基呋喃的甜度是蔗糖的30倍左右,因此也被認為是一種可能的甜味劑。[3]
參考資料
編輯- ^ P. A. Pavlov; Kul'nevich, V. G. Synthesis of 5-substituted furannitriles and their reaction with hydrazine. Khimiya Geterotsiklicheskikh Soedinenii. 1986, 2: 181–186.
- ^ Patrice Capdevielle; Lavigne, Andre; Maumy, Michel. Simple and efficient copper-catalyzed one-pot conversion of aldehydes into nitriles. Synthesis. 1989, 6: 451–452. doi:10.1055/s-1989-27285.
- ^ 3.0 3.1 Thomas J. Jennings, "Process for preparing furonitrile", US Patent 3,260,731 (1966)
- ^ Chenjie Zhu; Sun, Chengguo; Wei, Yunyang. Direct oxidative conversion of alcohols, aldehydes and amines into nitriles using hypervalent iodine(III) reagent. Synthesis. 2010, 24: 4235–4241. doi:10.1055/s-0030-1258281.
- ^ Bandgar, B. P.; Makone, S. S. Organic Reactions in Water: Transformation of Aldehydes to Nitriles using NBS under Mild Conditions. Synthetic Communications. 2006, 36 (10): 1347–1352. ISSN 0039-7911. doi:10.1080/00397910500522009.
- ^ Sachin S. Chaudhari; Akamanchi, Krishnacharya G. Thionyl chloride-benzotriazole: an efficient system for transformation of aldoximes to nitriles. Synthetic Communications. 1999, 29 (10): 1741–1745. doi:10.1080/00397919908086161.
- ^ A. Narsaiah; Sreenu, D.; Nagaiah, K. Triphenylphosphine-iodine. An efficient reagent system for the synthesis of nitriles from aldoximes. Synthetic Communications. 2006, 36 (2): 137–140. doi:10.1080/00397910500333225.
- ^ Aspinall, Helen C.; Beckingham, Oliver; Farrar, Michael D.; Greeves, Nicholas; Thomas, Christopher D. A general and convenient route to oxazolyl ligands. Tetrahedron Letters. 2011, 52 (40): 5120–5123. ISSN 0040-4039. doi:10.1016/j.tetlet.2011.07.070.
- ^ Jacqueline A. Campbell; McDougald, Graham; McNab, Hamish. Laboratory-scale synthesis of nitriles by catalyzed dehydration of amides and oximes under flash vacuum pyrolysis (FVP) conditions. Synthesis. 2007, 20: 3179–3184. doi:10.1055/s-2007-990782.