使用者:冰霜葵/沙盒叄
| 乙酸汞 | |||
|---|---|---|---|
| |||
| |||
| 英文名 | Mercury(II) acetate | ||
| 別名 | 乙酸高汞 乙酸汞(II)鹽 醋酸汞 二乙醯基氧基汞 雙(乙醯氧基)汞 乙酸汞(2+)鹽 二乙酸汞 mercuric acetate mercuriacetate | ||
| 識別 | |||
| CAS號 | 1600-27-7 | ||
| ChemSpider | 14599 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | BRMYZIKAHFEUFJ-NUQVWONBAS | ||
| 性質 | |||
| 化學式 | C4H6O4Hg | ||
| 莫耳質量 | 318.70 g·mol⁻¹ | ||
| 外觀 | 白色晶體或粉末[1] | ||
| 密度 | 3.27 g/cm³,固體 | ||
| 熔點 | 179℃(分解) | ||
| 溶解性(水) | 25 g/100 mL(10℃) | ||
| 溶解性 | 溶於乙醇 | ||
| 危險性 | |||
| 警示術語 | R:Template:R-p | ||
| 安全術語 | S:Template:S-p | ||
| NFPA 704 | |||
| 致死量或濃度: | |||
LD50(中位劑量)
|
76 mg/kg(大鼠經口) | ||
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |||
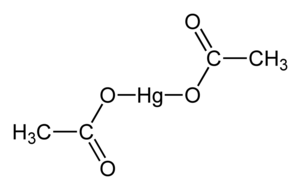
乙酸汞,化學式 Hg(O2CCH3)2 。通常縮寫成 Hg(OAc)2 ,this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.
性質
編輯Arenes undergo "mercuration" upon treatment with Hg(OAc)2. The one acetate group that remains on mercury can be displaced by chloride:[2]
- C6H5OH + Hg(OAc)2 → C6H4(OH)-2-HgOAc + HOAc
- C6H4(OH)-2-HgOAc + NaCl → C6H4(OH)-2-HgCl + NaOAc
The Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide. For example, treatment of methylacrylate with mercuric acetate in methanol gives an α-mercuri ester:[3]
- Hg(OAc)2 + CH2=CHCO2CH3 + CH3OH → CH3OCH2CH(HgOAc)CO2CH3 + HOAc
Mercury(II) has a high affinity for sulfur ligands. Hg(OAc)2 can be used as a reagent to remove the acetamidomethyl protecting group, which is used to "protect" thiol groups in organic synthesis. Similarly Hg(OAc)2 is a standard reagent to convert thiocarbonate esters into dithiocarbonates:
- (RS)2C=S + H2O + Hg(OAc)2 → (RS)2C=O + HgS + 2 HOAc
結構
編輯Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg.[4]
參考文獻
編輯- ^ (簡體中文)化工詞典 乙酸汞
- ^ Whitmore, F. C.; Hanson, E. R. "o-Chloromercuriphenol" Organic Syntheses, Collected Volume 1, p.161 (1941).http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0161
- ^ Carter, H. E.; West, H. D. 「dl-Serine」 Organic Syntheses, Collected Volume 3, p.774 (1955). http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV3P0774
- ^ R. Allmann. Z. Kristallogr., Kristallgeom., Kristallphys., Kristallchem. 1973, 138: 366–373. 缺少或
|title=為空 (幫助)


