视网膜母细胞瘤蛋白
在智人中發現的哺乳動物蛋白質
(重定向自RB1)
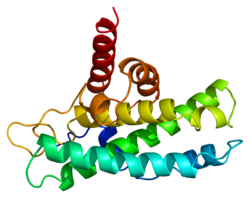
视网膜母细胞瘤蛋白(英语:retinoblastoma protein,常缩写为Rb、RB或RB1)又译为成视网膜细胞瘤蛋白是一种抑癌蛋白,并在几种主要的癌症发生时失活[6]。Rb的功能之一是通过抑制细胞周期进程直到细胞准备妥当来防止细胞过度生长。当细胞准备好要分裂时,Rb蛋白会被磷酸化为pRb而失去抑制活性,从而使细胞周期进行下去。
Rb是袋状蛋白家族的一员,其特点是有一个口袋状的结构用以结合其它蛋白[7][8]。使得一些致癌变蛋白,比如高危险群人类乳突病毒所感染的细胞产生的蛋白,结合并抑制pRb,最终导致癌症。
相互作用
编辑已知Rb蛋白与下列蛋白质有相互作用关系:
- Abl gene[9][10]
- Androgen receptor[11][12]
- Apoptosis-antagonizing transcription factor[13][14]
- ARID4A[15]
- 芳香烃受体[16]
- BRCA1[17][18][19]
- BRF1[20][21]
- C-jun[22]
- C-Raf[23][24]
- CDK9[25]
- CUTL1[26]
- 周期蛋白A1[27]
- 周期蛋白D1[28][29]
- 周期蛋白T2[25]
- DNMT1[30]
- E2F1[31][32][33][34][35][36][37]
- E2F2,[38]
- E4F1[34]
- EID1[39][40]
- ENC1[41]
- FRK[42]
- HBP1[43]
- HDAC1[15][44][45][46][47][48][49]
- HDAC3[15][50]
- Histone deacetylase 2[15]
- 胰岛素[51]
- JARID1A[52][53]
- LIN9[54]
- MCM7[55]
- MORF4L1[32][56]
- MRFAP1,[32][56]
- MyoD[57][58]
- NCOA6[59]
- PA2G4[60]
- 过氧化物酶体增殖物激活受体γ[50]
- PIK3R3[61]
- Plasminogen activator inhibitor-2[62]
- Polymerase (DNA directed), alpha 1[63]
- PRDM2[64]
- PRKRA[65]
- Prohibitin[24][66]
- 早幼粒细胞白血病蛋白[67]
- RBBP4[31][68]
- RBBP7[19][68]
- RBBP8[44][69]
- RBBP9[70]
- SNAPC1[71]
- SKP2[72][73]
- SNAPC3[71]
- SNW1[74]
- SUV39H1[75][76]
- TAF1[28][77][78][79]
- THOC1[80]
- TRAP1[81]
- TRIP11[82]
- UBTF[83]
- USP4.[84]
参考文献
编辑- ^ 與视网膜母细胞瘤蛋白相關的疾病;在維基數據上查看/編輯參考.
- ^ 2.0 2.1 2.2 GRCh38: Ensembl release 89: ENSG00000139687 - Ensembl, May 2017
- ^ 3.0 3.1 3.2 GRCm38: Ensembl release 89: ENSMUSG00000022105 - Ensembl, May 2017
- ^ Human PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Mouse PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Murphree AL, Benedict WF. Retinoblastoma: clues to human oncogenesis. Science. March 1984, 223 (4640): 1028–33. PMID 6320372. doi:10.1126/science.6320372.
- ^ Korenjak M, Brehm A. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Current Opinion in Genetics & Development. October 2005, 15 (5): 520–7 [2013-12-12]. PMID 16081278. doi:10.1016/j.gde.2005.07.001. (原始内容存档于2008-06-04).
- ^ Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. November 2002, 89 (2): 213–28 [2013-12-12]. PMID 12445661. doi:10.1016/S0168-1702(02)00190-9. (原始内容存档于2010-08-24).
- ^ Miyamura T, Nishimura J, Yufu Y, Nawata H. Interaction of BCR-ABL with the retinoblastoma protein in Philadelphia chromosome-positive cell lines. Int. J. Hematol. February 1997, 65 (2): 115–21. PMID 9071815. doi:10.1016/S0925-5710(96)00539-7.
- ^ Welch PJ, Wang JY. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell. November 1993, 75 (4): 779–90. PMID 8242749. doi:10.1016/0092-8674(93)90497-E.
- ^ Lu J, Danielsen M. Differential regulation of androgen and glucocorticoid receptors by retinoblastoma protein. J. Biol. Chem. November 1998, 273 (47): 31528–33. PMID 9813067. doi:10.1074/jbc.273.47.31528.
- ^ Yeh S, Miyamoto H, Nishimura K, Kang H, Ludlow J, Hsiao P, Wang C, Su C, Chang C. Retinoblastoma, a tumor suppressor, is a coactivator for the androgen receptor in human prostate cancer DU145 cells. Biochem. Biophys. Res. Commun. July 1998, 248 (2): 361–7. PMID 9675141. doi:10.1006/bbrc.1998.8974.
- ^ Bruno T, De Angelis R, De Nicola F, Barbato C, Di Padova M, Corbi N, Libri V, Benassi B, Mattei E, Chersi A, Soddu S, Floridi A, Passananti C, Fanciulli M. Che-1 affects cell growth by interfering with the recruitment of HDAC1 by Rb. Cancer Cell. November 2002, 2 (5): 387–99. PMID 12450794. doi:10.1016/S1535-6108(02)00182-4.
- ^ Fanciulli M, Bruno T, Di Padova M, De Angelis R, Iezzi S, Iacobini C, Floridi A, Passananti C. Identification of a novel partner of RNA polymerase II subunit 11, Che-1, which interacts with and affects the growth suppression function of Rb. FASEB J. May 2000, 14 (7): 904–12. PMID 10783144.
- ^ 15.0 15.1 15.2 15.3 Lai A, Lee JM, Yang WM, DeCaprio JA, Kaelin WG, Seto E, Branton PE. RBP1 Recruits Both Histone Deacetylase-Dependent and -Independent Repression Activities to Retinoblastoma Family Proteins. Mol. Cell. Biol. October 1999, 19 (10): 6632–41. PMC 84642 . PMID 10490602.
- ^ Ge NL, Elferink CJ. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J. Biol. Chem. August 1998, 273 (35): 22708–13. PMID 9712901. doi:10.1074/jbc.273.35.22708.
- ^ Aprelikova ON, Fang BS, Meissner EG, Cotter S, Campbell M, Kuthiala A, Bessho M, Jensen RA, Liu ET. BRCA1-associated growth arrest is RB-dependent. Proc. Natl. Acad. Sci. U.S.A. October 1999, 96 (21): 11866–71. PMC 18378 . PMID 10518542. doi:10.1073/pnas.96.21.11866.
- ^ Fan S, Yuan R, Ma YX, Xiong J, Meng Q, Erdos M, Zhao JN, Goldberg ID, Pestell RG, Rosen EM. Disruption of BRCA1 LXCXE motif alters BRCA1 functional activity and regulation of RB family but not RB protein binding. Oncogene. August 2001, 20 (35): 4827–41. PMID 11521194. doi:10.1038/sj.onc.1204666.
- ^ 19.0 19.1 Yarden RI, Brody LC. BRCA1 interacts with components of the histone deacetylase complex. Proc. Natl. Acad. Sci. U.S.A. April 1999, 96 (9): 4983–8. PMC 21803 . PMID 10220405. doi:10.1073/pnas.96.9.4983.
- ^ Johnston IM, Allison SJ, Morton JP, Schramm L, Scott PH, White RJ. CK2 Forms a Stable Complex with TFIIIB and Activates RNA Polymerase III Transcription in Human Cells. Mol. Cell. Biol. June 2002, 22 (11): 3757–68. PMC 133823 . PMID 11997511. doi:10.1128/MCB.22.11.3757-3768.2002.
- ^ Sutcliffe JE, Cairns CA, McLees A, Allison SJ, Tosh K, White RJ. RNA Polymerase III Transcription Factor IIIB Is a Target for Repression by Pocket Proteins p107 and p130. Mol. Cell. Biol. June 1999, 19 (6): 4255–61. PMC 104385 . PMID 10330166.
- ^ Nishitani J, Nishinaka T, Cheng CH, Rong W, Yokoyama KK, Chiu R. Recruitment of the retinoblastoma protein to c-Jun enhances transcription activity mediated through the AP-1 binding site. J. Biol. Chem. February 1999, 274 (9): 5454–61. PMID 10026157. doi:10.1074/jbc.274.9.5454.
- ^ Wang S, Ghosh RN, Chellappan SP. Raf-1 Physically Interacts with Rb and Regulates Its Function: a Link between Mitogenic Signaling and Cell Cycle Regulation. Mol. Cell. Biol. December 1998, 18 (12): 7487–98. PMC 109329 . PMID 9819434.
- ^ 24.0 24.1 Wang S, Nath N, Fusaro G, Chellappan S. Rb and Prohibitin Target Distinct Regions of E2F1 for Repression and Respond to Different Upstream Signals. Mol. Cell. Biol. November 1999, 19 (11): 7447–60. PMC 84738 . PMID 10523633.
- ^ 25.0 25.1 Simone C, Bagella L, Bellan C, Giordano A. Physical interaction between pRb and cdk9/cyclinT2 complex. Oncogene. June 2002, 21 (26): 4158–65. PMID 12037672. doi:10.1038/sj.onc.1205511.
- ^ Gupta S, Luong MX, Bleuming SA, Miele A, Luong M, Young D, Knudsen ES, Van Wijnen AJ, Stein JL, Stein GS. Tumor suppressor pRB functions as a co-repressor of the CCAAT displacement protein (CDP/cut) to regulate cell cycle controlled histone H4 transcription. J. Cell. Physiol. September 2003, 196 (3): 541–56. PMID 12891711. doi:10.1002/jcp.10335.
- ^ Yang R, Müller C, Huynh V, Fung YK, Yee AS, Koeffler HP. Functions of Cyclin A1 in the Cell Cycle and Its Interactions with Transcription Factor E2F-1 and the Rb Family of Proteins. Mol. Cell. Biol. March 1999, 19 (3): 2400–7. PMC 84032 . PMID 10022926.
- ^ 28.0 28.1 Siegert JL, Rushton JJ, Sellers WR, Kaelin WG, Robbins PD. Cyclin D1 suppresses retinoblastoma protein-mediated inhibition of TAFII250 kinase activity. Oncogene. November 2000, 19 (50): 5703–11. PMID 11126356. doi:10.1038/sj.onc.1203966.
- ^ Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A, Weinberg RA. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. May 1993, 73 (3): 499–511. PMID 8490963. doi:10.1016/0092-8674(93)90137-F.
- ^ Robertson KD, Ait-Si-Ali S, Yokochi T, Wade PA, Jones PL, Wolffe AP. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. July 2000, 25 (3): 338–42. PMID 10888886. doi:10.1038/77124.
- ^ 31.0 31.1 Nicolas E, Ait-Si-Ali S, Trouche D. The histone deacetylase HDAC3 targets RbAp48 to the retinoblastoma protein. Nucleic Acids Res. August 2001, 29 (15): 3131–6. PMC 55834 . PMID 11470869. doi:10.1093/nar/29.15.3131.
- ^ 32.0 32.1 32.2 Pardo PS, Leung JK, Lucchesi JC, Pereira-Smith OM. MRG15, a novel chromodomain protein, is present in two distinct multiprotein complexes involved in transcriptional activation. J. Biol. Chem. December 2002, 277 (52): 50860–6. PMID 12397079. doi:10.1074/jbc.M203839200.
- ^ Choubey D, Li SJ, Datta B, Gutterman JU, Lengyel P. Inhibition of E2F-mediated transcription by p202. EMBO J. October 1996, 15 (20): 5668–78. PMC 452311 . PMID 8896460.
- ^ 34.0 34.1 Fajas L, Paul C, Zugasti O, Le Cam L, Polanowska J, Fabbrizio E, Medema R, Vignais ML, Sardet C. pRB binds to and modulates the transrepressing activity of the E1A-regulated transcription factor p120E4F. Proc. Natl. Acad. Sci. U.S.A. July 2000, 97 (14): 7738–43. PMC 16614 . PMID 10869426. doi:10.1073/pnas.130198397.
- ^ Dyson N, Dembski M, Fattaey A, Ngwu C, Ewen M, Helin K. Analysis of p107-associated proteins: p107 associates with a form of E2F that differs from pRB-associated E2F-1. J. Virol. December 1993, 67 (12): 7641–7. PMC 238233 . PMID 8230483.
- ^ Wu CL, Zukerberg LR, Ngwu C, Harlow E, Lees JA. In vivo association of E2F and DP family proteins. Mol. Cell. Biol. May 1995, 15 (5): 2536–46. PMC 230484 . PMID 7739537.
- ^ Taniura H, Taniguchi N, Hara M, Yoshikawa K. Necdin, a postmitotic neuron-specific growth suppressor, interacts with viral transforming proteins and cellular transcription factor E2F1. J. Biol. Chem. January 1998, 273 (2): 720–8. PMID 9422723. doi:10.1074/jbc.273.2.720.
- ^ Lee C, Chang JH, Lee HS, Cho Y. Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor. Genes Dev. December 2002, 16 (24): 3199–212. PMC 187509 . PMID 12502741. doi:10.1101/gad.1046102.
- ^ Miyake S, Sellers WR, Safran M, Li X, Zhao W, Grossman SR, Gan J, DeCaprio JA, Adams PD, Kaelin WG. Cells Degrade a Novel Inhibitor of Differentiation with E1A-Like Properties upon Exiting the Cell Cycle. Mol. Cell. Biol. December 2000, 20 (23): 8889–902. PMC 86544 . PMID 11073989. doi:10.1128/MCB.20.23.8889-8902.2000.
- ^ MacLellan WR, Xiao G, Abdellatif M, Schneider MD. A Novel Rb- and p300-Binding Protein Inhibits Transactivation by MyoD. Mol. Cell. Biol. December 2000, 20 (23): 8903–15. PMC 86545 . PMID 11073990. doi:10.1128/MCB.20.23.8903-8915.2000.
- ^ Kim TA, Lim J, Ota S, Raja S, Rogers R, Rivnay B, Avraham H, Avraham S. NRP/B, a Novel Nuclear Matrix Protein, Associates With p110RB and Is Involved in Neuronal Differentiation. J. Cell Biol. May 1998, 141 (3): 553–66. PMC 2132755 . PMID 9566959. doi:10.1083/jcb.141.3.553.
- ^ Craven RJ, Cance WG, Liu ET. The nuclear tyrosine kinase Rak associates with the retinoblastoma protein pRb. Cancer Res. September 1995, 55 (18): 3969–72. PMID 7664264.
- ^ Lavender P, Vandel L, Bannister AJ, Kouzarides T. The HMG-box transcription factor HBP1 is targeted by the pocket proteins and E1A. Oncogene. June 1997, 14 (22): 2721–8. PMID 9178770. doi:10.1038/sj.onc.1201243.
- ^ 44.0 44.1 Dick FA, Sailhamer E, Dyson NJ. Mutagenesis of the pRB Pocket Reveals that Cell Cycle Arrest Functions Are Separable from Binding to Viral Oncoproteins. Mol. Cell. Biol. May 2000, 20 (10): 3715–27. PMC 85672 . PMID 10779361. doi:10.1128/MCB.20.10.3715-3727.2000.
- ^ Fuks F, Burgers WA, Brehm A, Hughes-Davies L, Kouzarides T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. January 2000, 24 (1): 88–91. PMID 10615135. doi:10.1038/71750.
- ^ Puri PL, Iezzi S, Stiegler P, Chen TT, Schiltz RL, Muscat GE, Giordano A, Kedes L, Wang JY, Sartorelli V. Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol. Cell. October 2001, 8 (4): 885–97. PMID 11684023. doi:10.1016/S1097-2765(01)00373-2.
- ^ Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. December 2002, 21 (55): 8388–96. PMID 12466959. doi:10.1038/sj.onc.1205944.
- ^ Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell. February 1998, 92 (4): 463–73. PMID 9491888. doi:10.1016/S0092-8674(00)80940-X.
- ^ Ferreira R, Magnaghi-Jaulin L, Robin P, Harel-Bellan A, Trouche D. The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase. Proc. Natl. Acad. Sci. U.S.A. September 1998, 95 (18): 10493–8. PMC 27922 . PMID 9724731. doi:10.1073/pnas.95.18.10493.
- ^ 50.0 50.1 Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril MB, Miard S, Auwerx J. The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation. Dev. Cell. December 2002, 3 (6): 903–10. PMID 12479814. doi:10.1016/S1534-5807(02)00360-X.
- ^ Radulescu RT, Bellitti MR, Ruvo M, Cassani G, Fassina G. Binding of the LXCXE Insulin Motif to a Hexapeptide Derived from Retinoblastoma Protein. Biochemical and Biophysical Research Communications. January 1995, 206 (1): 97–102. PMID 7818556. doi:10.1006/bbrc.1995.1014.
- ^ Chan SW, Hong W. Retinoblastoma-binding protein 2 (Rbp2) potentiates nuclear hormone receptor-mediated transcription. J. Biol. Chem. July 2001, 276 (30): 28402–12. PMID 11358960. doi:10.1074/jbc.M100313200.
- ^ Kim YW, Otterson GA, Kratzke RA, Coxon AB, Kaye FJ. Differential specificity for binding of retinoblastoma binding protein 2 to RB, p107, and TATA-binding protein. Mol. Cell. Biol. November 1994, 14 (11): 7256–64. PMC 359260 . PMID 7935440.
- ^ Gagrica S, Hauser S, Kolfschoten I, Osterloh L, Agami R, Gaubatz S. Inhibition of oncogenic transformation by mammalian Lin-9, a pRB-associated protein. EMBO J. November 2004, 23 (23): 4627–38. PMC 533054 . PMID 15538385. doi:10.1038/sj.emboj.7600470.
- ^ Sterner JM, Dew-Knight S, Musahl C, Kornbluth S, Horowitz JM. Negative Regulation of DNA Replication by the Retinoblastoma Protein Is Mediated by Its Association with MCM7. Mol. Cell. Biol. May 1998, 18 (5): 2748–57. PMC 110654 . PMID 9566894.
- ^ 56.0 56.1 Leung JK, Berube N, Venable S, Ahmed S, Timchenko N, Pereira-Smith OM. MRG15 activates the B-myb promoter through formation of a nuclear complex with the retinoblastoma protein and the novel protein PAM14. J. Biol. Chem. October 2001, 276 (42): 39171–8. PMID 11500496. doi:10.1074/jbc.M103435200.
- ^ Mal A, Sturniolo M, Schiltz RL, Ghosh MK, Harter ML. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program. EMBO J. April 2001, 20 (7): 1739–53. PMC 145490 . PMID 11285237. doi:10.1093/emboj/20.7.1739.
- ^ Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. February 1993, 72 (3): 309–24. PMID 8381715. doi:10.1016/0092-8674(93)90110-C.
- ^ Goo YH, Na SY, Zhang H, Xu J, Hong S, Cheong J, Lee SK, Lee JW. Interactions between activating signal cointegrator-2 and the tumor suppressor retinoblastoma in androgen receptor transactivation. J. Biol. Chem. February 2004, 279 (8): 7131–5. PMID 14645241. doi:10.1074/jbc.M312563200.
- ^ Xia X, Cheng A, Lessor T, Zhang Y, Hamburger AW. Ebp1, an ErbB-3 binding protein, interacts with Rb and affects Rb transcriptional regulation. J. Cell. Physiol. May 2001, 187 (2): 209–17. PMID 11268000. doi:10.1002/jcp.1075.
- ^ Xia X, Cheng A, Akinmade D, Hamburger AW. The N-Terminal 24 Amino Acids of the p55 Gamma Regulatory Subunit of Phosphoinositide 3-Kinase Binds Rb and Induces Cell Cycle Arrest. Mol. Cell. Biol. March 2003, 23 (5): 1717–25. PMC 151709 . PMID 12588990. doi:10.1128/MCB.23.5.1717-1725.2003.
- ^ Darnell GA, Antalis TM, Johnstone RW, Stringer BW, Ogbourne SM, Harrich D, Suhrbier A. Inhibition of Retinoblastoma Protein Degradation by Interaction with the Serpin Plasminogen Activator Inhibitor 2 via a Novel Consensus Motif. Mol. Cell. Biol. September 2003, 23 (18): 6520–32. PMC 193706 . PMID 12944478. doi:10.1128/MCB.23.18.6520-6532.2003.
- ^ Takemura M, Kitagawa T, Izuta S, Wasa J, Takai A, Akiyama T, Yoshida S. Phosphorylated retinoblastoma protein stimulates DNA polymerase alpha. Oncogene. November 1997, 15 (20): 2483–92. PMID 9395244. doi:10.1038/sj.onc.1201431.
- ^ Buyse IM, Shao G, Huang S. The retinoblastoma protein binds to RIZ, a zinc-finger protein that shares an epitope with the adenovirus E1A protein. Proc. Natl. Acad. Sci. U.S.A. May 1995, 92 (10): 4467–71. PMC 41965 . PMID 7538672. doi:10.1073/pnas.92.10.4467.
- ^ Simons A, Melamed-Bessudo C, Wolkowicz R, Sperling J, Sperling R, Eisenbach L, Rotter V. PACT: cloning and characterization of a cellular p53 binding protein that interacts with Rb. Oncogene. January 1997, 14 (2): 145–55. PMID 9010216. doi:10.1038/sj.onc.1200825.
- ^ Wang S, Nath N, Adlam M, Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. June 1999, 18 (23): 3501–10. PMID 10376528. doi:10.1038/sj.onc.1202684.
- ^ Alcalay M, Tomassoni L, Colombo E, Stoldt S, Grignani F, Fagioli M, Szekely L, Helin K, Pelicci PG. The Promyelocytic Leukemia Gene Product (PML) Forms Stable Complexes with the Retinoblastoma Protein. Mol. Cell. Biol. February 1998, 18 (2): 1084–93. PMC 108821 . PMID 9448006.
- ^ 68.0 68.1 Qian YW, Lee EY. Dual retinoblastoma-binding proteins with properties related to a negative regulator of ras in yeast. J. Biol. Chem. October 1995, 270 (43): 25507–13. PMID 7503932. doi:10.1074/jbc.270.43.25507.
- ^ Fusco C, Reymond A, Zervos AS. Molecular cloning and characterization of a novel retinoblastoma-binding protein. Genomics. August 1998, 51 (3): 351–8. PMID 9721205. doi:10.1006/geno.1998.5368.
- ^ Woitach JT, Zhang M, Niu CH, Thorgeirsson SS. A retinoblastoma-binding protein that affects cell-cycle control and confers transforming ability. Nat. Genet. August 1998, 19 (4): 371–4. PMID 9697699. doi:10.1038/1258.
- ^ 71.0 71.1 Hirsch HA, Gu L, Henry RW. The Retinoblastoma Tumor Suppressor Protein Targets Distinct General Transcription Factors To Regulate RNA Polymerase III Gene Expression. Mol. Cell. Biol. December 2000, 20 (24): 9182–91. PMC 102176 . PMID 11094070. doi:10.1128/MCB.20.24.9182-9191.2000.
- ^ Ji P, Jiang H, Rekhtman K, Bloom J, Ichetovkin M, Pagano M, Zhu L. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol. Cell. October 2004, 16 (1): 47–58. PMID 15469821. doi:10.1016/j.molcel.2004.09.029.
- ^ Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J, Sellers RS, Nakayama K, Nakayama KI, Cobrinik D, Zhu L. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/- mice. Nat. Genet. January 2010, 42 (1): 83–8. PMC 2990528 . PMID 19966802. doi:10.1038/ng.498.
- ^ Prathapam T, Kühne C, Banks L. Skip interacts with the retinoblastoma tumor suppressor and inhibits its transcriptional repression activity. Nucleic Acids Res. December 2002, 30 (23): 5261–8. PMC 137971 . PMID 12466551. doi:10.1093/nar/gkf658.
- ^ Nielsen SJ, Schneider R, Bauer UM, Bannister AJ, Morrison A, O'Carroll D, Firestein R, Cleary M, Jenuwein T, Herrera RE, Kouzarides T. Rb targets histone H3 methylation and HP1 to promoters. Nature. August 2001, 412 (6846): 561–5. PMID 11484059. doi:10.1038/35087620.
- ^ Vandel L, Nicolas E, Vaute O, Ferreira R, Ait-Si-Ali S, Trouche D. Transcriptional Repression by the Retinoblastoma Protein through the Recruitment of a Histone Methyltransferase. Mol. Cell. Biol. October 2001, 21 (19): 6484–94. PMC 99795 . PMID 11533237. doi:10.1128/MCB.21.19.6484-6494.2001.
- ^ Shao Z, Ruppert S, Robbins PD. The retinoblastoma-susceptibility gene product binds directly to the human TATA-binding protein-associated factor TAFII250. Proc. Natl. Acad. Sci. U.S.A. April 1995, 92 (8): 3115–9. PMC 42115 . PMID 7724524. doi:10.1073/pnas.92.8.3115.
- ^ Siegert JL, Robbins PD. Rb Inhibits the Intrinsic Kinase Activity of TATA-Binding Protein-Associated Factor TAFII250. Mol. Cell. Biol. January 1999, 19 (1): 846–54. PMC 83941 . PMID 9858607.
- ^ Shao Z, Siegert JL, Ruppert S, Robbins PD. Rb interacts with TAF(II)250/TFIID through multiple domains. Oncogene. July 1997, 15 (4): 385–92. PMID 9242374. doi:10.1038/sj.onc.1201204.
- ^ Durfee T, Mancini MA, Jones D, Elledge SJ, Lee WH. The amino-terminal region of the retinoblastoma gene product binds a novel nuclear matrix protein that co-localizes to centers for RNA processing. J. Cell Biol. November 1994, 127 (3): 609–22. PMC 2120229 . PMID 7525595. doi:10.1083/jcb.127.3.609.
- ^ Chen CF, Chen Y, Dai K, Chen PL, Riley DJ, Lee WH. A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock. Mol. Cell. Biol. September 1996, 16 (9): 4691–9. PMC 231469 . PMID 8756626.
- ^ Chang KH, Chen Y, Chen TT, Chou WH, Chen PL, Ma YY, Yang-Feng TL, Leng X, Tsai MJ, O'Malley BW, Lee WH. A thyroid hormone receptor coactivator negatively regulated by the retinoblastoma protein. Proc. Natl. Acad. Sci. U.S.A. August 1997, 94 (17): 9040–5. PMC 23019 . PMID 9256431. doi:10.1073/pnas.94.17.9040.
- ^ Hannan KM, Hannan RD, Smith SD, Jefferson LS, Lun M, Rothblum LI. Rb and p130 regulate RNA polymerase I transcription: Rb disrupts the interaction between UBF and SL-1. Oncogene. October 2000, 19 (43): 4988–99. PMID 11042686. doi:10.1038/sj.onc.1203875.
- ^ Blanchette P, Gilchrist CA, Baker RT, Gray DA. Association of UNP, a ubiquitin-specific protease, with the pocket proteins pRb, p107 and p130. Oncogene. September 2001, 20 (39): 5533–7. PMID 11571651. doi:10.1038/sj.onc.1204823.
视网膜母细胞瘤蛋白引用了美国国家医学图书馆提供的数据,这些数据属于公共领域。
延伸阅读
编辑- Momand J, Wu HH, Dasgupta G. MDM2—master regulator of the p53 tumor suppressor protein. Gene. 2000, 242 (1–2): 15–29. PMID 10721693. doi:10.1016/S0378-1119(99)00487-4.
- Zheng L, Lee WH. Retinoblastoma tumor suppressor and genome stability. Adv. Cancer Res. Advances in Cancer Research. 2003, 85: 13–50. ISBN 978-0-12-006685-8. PMID 12374284. doi:10.1016/S0065-230X(02)85002-3.
- Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nature Reviews Cancer. 2003, 2 (12): 910–7. PMID 12459729. doi:10.1038/nrc950.
- Lai H, Ma F, Lai S. Identification of the novel role of pRB in eye cancer. J. Cell. Biochem. 2003, 88 (1): 121–7. PMID 12461781. doi:10.1002/jcb.10283.
- Simin K, Wu H, Lu L; et al. pRb Inactivation in Mammary Cells Reveals Common Mechanisms for Tumor Initiation and Progression in Divergent Epithelia. PLoS Biol. 2006, 2 (2): E22. PMC 340938 . PMID 14966529. doi:10.1371/journal.pbio.0020022.
- Lohmann DR, Gallie BL. Retinoblastoma: revisiting the model prototype of inherited cancer. American Journal of Medical Genetics. 2004, 129 (1): 23–8. PMID 15264269. doi:10.1002/ajmg.c.30024.
- Clemo NK, Arhel NJ, Barnes JD; et al. The role of the retinoblastoma protein (Rb) in the nuclear localization of BAG-1: implications for colorectal tumour cell survival. Biochem. Soc. Trans. 2005, 33 (Pt 4): 676–8. PMID 16042572. doi:10.1042/BST0330676.
- Rodríguez-Cruz M, del Prado M, Salcedo M. [Genomic retinoblastoma perspectives: implications of tumor supressor gene RB1]. Rev. Invest. Clin. 2006, 57 (4): 572–81. PMID 16315642.
- Knudsen ES, Knudsen KE. Retinoblastoma tumor suppressor: where cancer meets the cell cycle. Exp. Biol. Med. (Maywood). 2006, 231 (7): 1271–81. PMID 16816134.
外部链接
编辑- 医学主题词表(MeSH):RB1+protein,+human
- 医学主题词表(MeSH):Retinoblastoma+genes
- GeneReviews/NIH/NCBI/UW entry on Retinoblastoma (页面存档备份,存于互联网档案馆)
- Retinoblastoma Genetics (页面存档备份,存于互联网档案馆)
- Drosophila Retinoblastoma-family protein - The Interactive Fly (页面存档备份,存于互联网档案馆)
- Drosophila Retinoblastoma-family protein 2 - The Interactive Fly (页面存档备份,存于互联网档案馆)
- Evolutionary Homologs Retinoblastoma-family proteins - The Interactive Fly (页面存档备份,存于互联网档案馆)
- There is a diagram of the pRb-E2F interactions here[永久失效链接].