阿贝卡尔
化合物
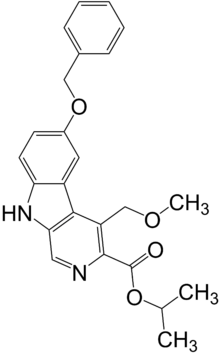
阿贝卡尔(英语:Abecarnil,代号:ZK-112,119)是一种来自β-咔啉家族的抗焦虑药。它是相对较新开发的一类药物之一,称为非苯二氮䓬类药物,与较早的苯二氮䓬类药物具有相似的作用,但化学结构截然不同。它是一种部分激动剂,选择性地作用于GABAA受体的苯二氮䓬位点。[1]
 | |
| 临床资料 | |
|---|---|
| ATC码 |
|
| 药物动力学数据 | |
| 生物半衰期 | 3.4 hours (IV), 7 hours (oral) |
| 识别信息 | |
| |
| CAS号 | 111841-85-1 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
| 化学式 | C24H24N2O4 |
| 摩尔质量 | 404.47 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
阿贝卡尔最初是作为抗焦虑药物开发的,但尚未商业化开发用于人类,目前主要用于研究开发其他新型镇静和抗焦虑药物。对其作用的调查仍在继续,它看起来很可能被开发用于治疗焦虑症,[2]以及作为一种不太容易上瘾的替代药物来治疗苯二氮䓬类药物[3]和酒精[4]成瘾。与非选择性全激动剂苯二氮䓬类药物相比,阿贝卡尼的耐受性和戒断问题也可能更少。[5]
阿贝卡尔是一种相对亚型选择性的药物,主要产生抗焦虑作用,具有相对较弱的镇静或肌肉松弛作用,[6][7]并且不会显着增强酒精的作用。[8]
参见
编辑参考资料
编辑- ^ Ozawa, M; Nakada, Y; Sugimachi, K; Yabuuchi, F; Akai, T; Mizuta, E; Kuno, S; Yamaguchi, M. Pharmacological characterization of the novel anxiolytic beta-carboline abecarnil in rodents and primates. Japanese Journal of Pharmacology. Mar 1994, 64 (3): 179–87. PMID 7912751. doi:10.1254/jjp.64.179 .
- ^ Aufdembrinke B. Abecarnil, a new beta-carboline, in the treatment of anxiety disorders. British Journal of Psychiatry. 1998, 34 (34): 55–63. PMID 9829018. S2CID 24311570. doi:10.1192/S0007125000293537.
- ^ Pinna G, Galici R, Schneider HH, Stephens DN, Turski L. Alprazolam dependence prevented by substituting with the beta-carboline abecarnil. Proceedings of the National Academy of Sciences of the United States of America. Mar 1997, 94 (6): 2719–23. Bibcode:1997PNAS...94.2719P. PMC 20156 . PMID 9122263. doi:10.1073/pnas.94.6.2719 .
- ^ Jung ME, Wallis CJ, Gatch MB, Lal H. Abecarnil and alprazolam reverse anxiety-like behaviors induced by ethanol withdrawal. Alcohol. Jun 2000, 21 (2): 161–8. PMID 10963939. doi:10.1016/S0741-8329(00)00079-3.
- ^ Löscher W, Hönack D. Withdrawal precipitation by benzodiazepine receptor antagonists in dogs chronically treated with diazepam or the novel anxiolytic and anticonvulsant beta-carboline abecarnil. Naunyn Schmiedebergs Arch. Pharmacol. April 1992, 345 (4): 452–60. PMID 1352384. S2CID 20486955. doi:10.1007/BF00176624.
- ^ Krause W, Schutt B, Duka T. Pharmacokinetics and acute toleration of the beta-carboline derivative abecarnil in man. Arzneimittelforschung. May 1990, 40 (5): 529–32. PMID 1974428.
- ^ Duka T, Schutt B, Krause W, Dorow R, McDonald S, Fichte K. Human studies on abecarnil, a new beta-carboline anxiolytic: safety, tolerability and preliminary pharmacological profile. British Journal of Clinical Pharmacology. Apr 1993, 35 (4): 386–94. PMC 1381549 . PMID 8097921. doi:10.1111/j.1365-2125.1993.tb04155.x.
- ^ Stephens DN, Schneider HH, Kehr W, Andrews JS, Rettig KJ, Turski L, Schmiechen R, Turner JD, Jensen LH, et al. Abecarnil, a metabolically stable, anxioselective beta-carboline acting at benzodiazepine receptors. Journal of Pharmacology and Experimental Therapeutics. Apr 1990, 253 (1): 334–43. PMID 1970361.
- ^ Sannerud CA, Ator NA, Griffiths RR. Behavioral pharmacology of abecarnil in baboons: self-injection, drug discrimination and physical dependence. Behavioural Pharmacology. Oct 1992, 3 (5): 507–516. PMID 11224153. S2CID 32081258. doi:10.1097/00008877-199210000-00009.
- ^ Ballenger JC, McDonald S, Noyes R, Rickels K, Sussman N, Woods S, Patin J, Singer J. The first double-blind, placebo-controlled trial of a partial benzodiazepine agonist abecarnil (ZK 112-119) in generalized anxiety disorder. Psychopharmacology Bulletin. 1991, 27 (2): 171–9. PMID 1681563.