黄芩苷
化合物
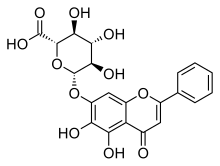
黄芩苷(英语:Baicalin)是一种黄酮类化合物,是黄芩素(Baicalein)与葡萄糖醛酸形成的糖苷,提取自黄芩(Scutellaria baicalensis)[1]、盔状黄芩(Scutellaria galericulata)[2]等植物。
| 黄芩苷 | |
|---|---|
| |
| IUPAC名 (2S,3S,4S,5R,6S)-6-[(5,6-Dihydroxy-4-oxo-2-phenyl-4H-1-benzopyran-7-yl)oxy]-3,4,5-trihydroxyoxane-2-carboxylic acid | |
| 别名 | 黄芩素 7-O-葡萄糖醛酸 |
| 识别 | |
| CAS号 | 21967-41-9 |
| PubChem | 64982 |
| ChemSpider | 58507 |
| SMILES |
|
| InChI |
|
| InChIKey | IKIIZLYTISPENI-ZFORQUDYBD |
| Beilstein | 70480 |
| ChEBI | 2981 |
| KEGG | C10025 |
| 性质 | |
| 化学式 | C21H18O11 |
| 摩尔质量 | 446.36 g·mol−1 |
| 熔点 | 202—205 °C(396—401 °F;475—478 K) |
| 危险性 | |
GHS危险性符号
| |
| GHS提示词 | Warning |
| H-术语 | H315, H319, H335 |
| P-术语 | P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
药用价值
编辑黄芩苷及其糖苷配基黄芩素都是苯二氮䓬受体的正别构调节剂。[4][5][6]小鼠实验表明黄芩苷是抗焦虑药,但不是镇静剂或肌肉松弛剂。[7][8]黄芩和盔状黄芩的抗焦虑作用可能源自黄芩苷及其它黄酮类化合物。[9][10]
参考文献
编辑- ^ 1.0 1.1 Su, Hai-xia; Yao, Sheng; Zhao, Wen-Feng; Li, Min-jun; Liu, Jia; Shang, Wei-Juan; Xie, Hang; Ke, Chang-Qiang; Hu, Hang-Chen; Gao, Mei-na; Yu, Kun-Qian; Liu, Hong; Shen, Jing-Shan; Tang, Wei; Zhang, Lei-ke; Xiao, Geng-fu; Ni, Li; Wang, Dao-wen; Zuo, Jian-Ping; Jiang, Hua-Liang; Bai, Fang; Wu, Yan; Ye, Yang; Xu, Ye-Chun. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacologica Sinica. 2020, 41 (9): 1167–1177. PMC 7393338 . PMID 32737471. doi:10.1038/s41401-020-0483-6.
- ^ P.H. and Horhammer, L., Hager's Handbuch der Pharmazeutischen Praxis, Vols. 2-6, Springer-Verlag, Berlin, 1969-1979
- ^ Taira, Zenei; Yabe, Kazunori; Hamaguchi, Yuka; Hirayama, Kensuke; Kishimoto, Makoto; Ishida, Shiro; Ueda, Yukari. Effects of Sho-saiko-to extract and its components, Baicalin, baicalein, glycyrrhizin and glycyrrhetic acid, on pharmacokinetic behavior of salicylamide in carbon tetrachloride intoxicated rats. Food and Chemical Toxicology (Elsevier BV). 2004, 42 (5): 803–807. ISSN 0278-6915. doi:10.1016/j.fct.2003.12.017.
- ^ Wang H, Hui KM, Xu S, Chen Y, Wong JT, Xue H. Two flavones from Scutellaria baicalensis Georgi and their binding affinities to the benzodiazepine site of the GABAA receptor complex. Pharmazie. 2002, 57 (12): 857–8. PMID 12561253.
- ^ Hui KM, Wang XH, Xue H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med. 2000, 66 (1): 91–3. PMID 10705749. S2CID 260249283. doi:10.1055/s-0029-1243121.
- ^ Edwin Lowell Cooper; Nobuo Yamaguchi. Complementary and Alternative Approaches to Biomedicine. Springer Science & Business Media. 1 January 2004: 188–. ISBN 978-0-306-48288-5.
- ^ Xu Z, Wang F, Tsang SY, Ho KH, Zheng H, Yuen CT, Chow CY, Xue H. Anxiolytic-Like Effect of baicalin and its additivity with other anxiolytics. Planta Med. 2006, 72 (2): 189–92. PMID 16491459. S2CID 2398014. doi:10.1055/s-2005-873193.
- ^ Liao JF, Hung WY, Chen CF. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur. J. Pharmacol. 2003, 464 (2–3): 141–6. PMID 12620506. doi:10.1016/s0014-2999(03)01422-5.
- ^ Awad R, Arnason JT, Trudeau V, Bergeron C, Budzinski JW, Foster BC, Merali Z. Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): a medicinal plant with anxiolytic properties. Phytomedicine. 2003, 10 (8): 640–9. PMID 14692724. doi:10.1078/0944-7113-00374.
- ^ Stefanie Schwartz. Psychoactive Herbs in Veterinary Behavior Medicine. John Wiley & Sons. 9 January 2008: 139–. ISBN 978-0-470-34434-7.
- ^ Tarragó, T; Kichik, N; Claasen, B; Prades, R; Teixidó, M; Giralt, E. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorganic & Medicinal Chemistry. 2008, 16 (15): 7516–24. PMID 18650094. doi:10.1016/j.bmc.2008.04.067.
- ^ Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, Hines OJ, Gukovskaya AS, Go VL, Eibl G. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011, 1813 (8): 1465–1474. PMC 3123440 . PMID 21596068. doi:10.1016/j.bbamcr.2011.05.003.