利米洛韋
化合物
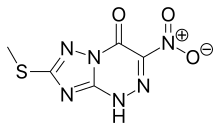
利米洛韋(INN:Riamilovir ),又稱三氮唑核苷(Triazavirin)[1],是一種廣譜抗病毒藥物。由俄羅斯烏拉爾聯邦大學、俄羅斯科學院、烏拉爾生物製藥中心和麥德森製藥(Medsintez)聯合開發[2]。該藥物中含有三唑三嗪結構,是一種非核苷酸抗病毒藥物新結構類型[3]。
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | TZV, Triazavirin |
| ATC碼 |
|
| 識別資訊 | |
| |
| CAS號 | 123606-06-4 928659-17-0 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.074 |
| 化學資訊 | |
| 化學式 | C5H4N6O3S |
| 摩爾質量 | 228.19 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
利米洛韋的主要通過合成嘌呤核苷酸鹼基類似物來抑制病毒RNA基因片段的複製,由此達到抑制病毒繁殖的效果[4][5][6]。
用途
編輯利米洛韋最初是作為抗諸如H5N1等流感病毒的藥物而研發的,早期已經有許多關於抗流感病毒效果的測試[6][4][7][8][9]。隨後又發現其對如蜱傳腦炎病毒[5][10]等其他病毒亦有效果,同時也針對高危型流感病毒感染、流感後肺部二次細菌感染[11]等疾病以及拉沙熱、伊波拉等致命病毒性疾病的治療效果進行研究[12][13][14][15][16]。 利米洛韋目前已經通過了臨床測試,並對急性呼吸道病毒感染展現出抗病毒活性[17][18][19][20]。
2020年,俄羅斯,中國和南非三國開展了利米洛韋抗新冠病毒(SARS-CoV-2)活性的研究[21][22][23][24][25]。利米洛韋的作用機制仍存在爭議。迄今尚未有關於利米洛韋對SARS-CoV-2或流感靶蛋白活性的論文發表[26]。
2014年8月,俄羅斯衛生部通過了利米洛韋的註冊登記,可憑處方即可購買[2]。同時利米洛韋的註冊程序已在南非開展[24]。
批評
編輯一篇論文批評利米洛韋的研發過程沒有符合雙盲實驗和隨機化受試對象要求,並且受試者僅有66人,其中有44人為對照組[17]。
參考文獻
編輯- ^ 中国初步证实俄罗斯药物三氮唑核苷抗击新冠病毒的疗效. 俄羅斯衛星通訊社. 2020年9月11日 [2024-07-09].
- ^ 2.0 2.1 Triazaverin Is Officially Recommended. www.medsintez.com. [2021-02-25].
- ^ Rusinov VL, Sapozhnikova IM, Ulomskii EN, Medvedeva NR, Egorov VV, Kiselev OI, Deeva EG, Vasin AV, Chupakhin ON. Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteine-containing proteins.. Chemistry of Heterocyclic Compounds. 2015, 51 (3): 275–280. S2CID 83702396. doi:10.1007/s10593-015-1695-4 .
- ^ 4.0 4.1 Karpenko I, Deev S, Kiselev O, Charushin V, Rusinov V, Ulomsky E, Deeva E, Yanvarev D, Ivanov A, Smirnova O, Kochetkov S, Chupakhin O, Kukhanova M. Antiviral properties, metabolism, and pharmacokinetics of a novel azolo-1,2,4-triazine-derived inhibitor of influenza A and B virus replication. Antimicrobial Agents and Chemotherapy. May 2010, 54 (5): 2017–2022. PMC 2863629 . PMID 20194696. doi:10.1128/AAC.01186-09.
- ^ 5.0 5.1 Loginova SI, Borisevich SV, Rusinov VL, Ulomskiĭ UN, Charushin VN, Chupakhin ON. [Investigation of Triazavirin antiviral activity against tick-borne encephalitis pathogen in cell culture]. Antibiotiki i Khimioterapiia. 2014, 59 (1–2): 3–5. PMID 25051708 (俄語).
- ^ 6.0 6.1 Loginova SI, Borisevich SV, Maksimov VA, Bondarev VP, Kotovskaia SK, Rusinov VL, Charushin VN. [Investigation of triazavirin antiviral activity against influenza A virus (H5N1) in cell culture]. Antibiotiki i Khimioterapiia. 2007, 52 (11–12): 18–20. PMID 19275052 (俄語).
- ^ Kiselev OI, Deeva EG, Mel'nikova TI, Kozeletskaia KN, Kiselev AS, Rusinov VL, Charushin VN, Chupakhin ON. [A new antiviral drug Triazavirin: results of phase II clinical trial]. Voprosy Virusologii. 2012, 57 (6): 9–12. PMID 23477247 (俄語).
- ^ Kasianenko KV, Lvov NI, Maltsev OV, Zhdanov KV. Nucleoside analogues for the treatment of influenza: History and experience. Journal of Infectology. 9 October 2019, 11 (3): 20–26. S2CID 241604614. doi:10.22625/2072-6732-2019-11-3-20-26 (俄語).
- ^ Sologub TV, Tokin II, Midikari AS, Tsvetkov VV. A comparative efficacy and safety of using antiviral drugs in therapy of patients with influenza. Infekcionnye Bolezni. 2017, 15 (3): 25–32. doi:10.20953/1729-9225-2017-3-25-32 (俄語).
- ^ Loginova SY, Borisevich SV, Rusinov VL, Ulomsky EN, Charushin VN, Chupakhin ON, Sorokin PV. [Investigation of Therapeutic Efficacy of Triazavirin Against Experimental Forest-Spring Encephalitis on Albino Mice]. Antibiotiki i Khimioterapiia. 2015, 60 (7–8): 11–13. PMID 26863736 (俄語).
- ^ Leneva IA, Falynskova IN, Makhmudova NR, Glubokova EA, Kartashova NP, Leonova EI, Mikhailova NA, Shestakova IV. Effect of triazavirine on the outcome of a lethal influenza infection and secondary bacterial pneumonia following influenza in mice. Microbiology Independent Research Journal. 2018-02-22, 4 (1). doi:10.18527/2500-2236-2017-4-1-52-57 .
- ^ Target: Ebola. Pravda. 2014-12-22 [18 January 2015].
- ^ Yekaterinburg pharmacies to sell domestic antiviral drug. Yekaterinburg News Reports. 6 January 2015 [18 January 2015]. (原始內容存檔於18 January 2015).
- ^ Cox S. Ebola crisis: Vaccine 'too late' for outbreak. BBC News, 17 October 2014. BBC News. 2014-10-17.
- ^ Bora K. Russia Will Begin Testing Triazavirin, Used For Lassa Fever, And Other Drugs On Ebola: Health Ministry.. International Business Times. 12 November 2014.
- ^ Kezina D. New antiviral drug from Urals will help fight Ebola and other viruses.. Russia Beyond the Headlines. 12 November 2014.
- ^ 17.0 17.1 Tikhonova EP, Kuz'mina TY, Andronova NV, Tyushevskaya OA, Elistratova TA, Kuz'min AE. Study of effectiveness of antiviral drugs (umifenovir, triazavirin) against acute respiratory viral infections. Kazan Medical Journal. 2018-04-15, 99 (2): 215–223. ISSN 2587-9359. doi:10.17816/KMJ2018-215 (俄語).
- ^ Verevshchikov VK, Shemyakina EK, Sabitov AU, Khamanova YB. The Possibilities of Etiotropic Therapy for Influenza and ARVI with Taking into Account the Period of Hospitalization and the Risk of Developing Secondary Complications. Antibiotics and Chemotherapy. 2019 [2021-02-25].
- ^ Lioznov DA, Tokin II, Zubkova TG, Sorokin PV. [The practice of using a domestic antiviral drug in the etiotropic therapy of acute respiratory viral infection]. Terapevticheskii Arkhiv. December 2020, 92 (12): 160–164. PMID 33720589. doi:10.26442/00403660.2020.12.200427 (俄語).
- ^ Verevshchikov VK, Shemyakina EK, Sabitov AU, Batskalevich NA. Modern Etiotropic Therapy of Influenza and ARVI in Adult Patients with Premorbid Pathology. Antibiotics and Chemotherapy. 2018 [2021-02-25].
- ^ Phiri C. China Tests Russian Antiviral Drug Which Might Treat Coronavirus As Moscow Warns Of Possible 'Mass Outbreak'. Zambia Reports. 5 February 2020 [11 February 2020]. (原始內容存檔於5 February 2020).
- ^ Wu X, Yu K, Wang Y, Xu W, Ma H, Hou Y, Li Y, Cai B, Zhu L, Zhang M, Hu X, Gao J, Wang Y, Qin H, Wang W, Zhao M, Wu X, Zhang Y, Li L, Li K, Du Z, Mol BW, Yang B. Efficacy and Safety of Triazavirin Therapy for Coronavirus Disease 2019: A Pilot Randomized Controlled Trial. Engineering. October 2020, 6 (10): 1185–1191. PMC 7476906 . PMID 32923016. S2CID 221520816. doi:10.1016/j.eng.2020.08.011 .
- ^ Brandt K. SA study to trial antiviral Triazavirin as COVID-19 treatment. ewn.co.za. [2021-02-25] (英語).
- ^ 24.0 24.1 Discussion on Russia's antiviral drug Triazavirin. SABC News. 21 January 2021 –透過YouTube.
- ^ Sabitov AU, Belousov VV, Edin AS, Oleinichenko EV, Gladunova EP, Tikhonova EP, Kuzmina TY, Kalinina YS, Sorokin PV. Practical Experience of Using Riamilovir in Treatment of Patients with Moderate COVID-19. Antibiotiki i Khimioterapiya. 21 November 2020, 65 (7–8): 27–30. S2CID 229508119. doi:10.37489/0235-2990-2020-65-7-8-27-30 .
- ^ Chupakhin ON, Rusinov VL, Varaksin MV, Ulomskiy EN, Savateev KV, Butorin II, Du W, Sun Z, Charushin VN. Triazavirin-A Novel Effective Antiviral Drug. International Journal of Molecular Sciences. November 2022, 23 (23): 14537. PMC 9738222 . PMID 36498864. doi:10.3390/ijms232314537 .