環氯胍
化合物
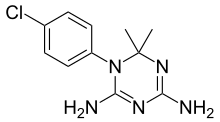
環氯胍(英語:Cycloguanil),又稱氯胍三嗪[1]或氯苯三嗪[2],化學上是一種胺縮醛,藥理學上屬二氫葉酸還原酶抑制劑[3],是抗瘧疾藥物氯胍的經肝臟細胞色素P450代謝產物,並被認為是氯胍在體內發揮抗瘧作用的活性成分(即氯胍是環氯胍的前體藥物)[1][4]。然而最新研究表明,在抗瘧藥物馬拉隆(Malarone,即複方氯胍/阿托伐醌)中,氯胍與阿托伐醌雖然有協同作用,但代謝成環氯胍後對阿托伐醌是拮抗的,表明氯胍與環氯胍不同,除了抑制二氫葉酸還原酶之外,可能還具備另一種抗瘧作用機制[5]。
 | |
 | |
| 臨床資料 | |
|---|---|
| ATC碼 | |
| 識別資訊 | |
| |
| CAS號 | 516-21-2 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化學資訊 | |
| 化學式 | C11H14ClN5 |
| 摩爾質量 | 251.72 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
儘管環氯胍目前並未被廣泛用作抗瘧藥,但隨着現有抗瘧藥物的耐藥性不斷增強,人們對環胍與其他藥物聯合使用的研究重新產生了興趣[6]。
合成
編輯環氯胍製備路線如圖,採用對氯苯胺(1)與雙氰胺(2)反應得到對氯苯基雙胍 (3),對氯苯基雙胍在丙酮作用下發生縮合即可得到環氯胍。
參考文獻
編輯- ^ 1.0 1.1 盧愛華,舒焱,黃松林,等. 体外中国成人肝微粒体中氯胍活化为氯胍三嗪由CYP2C19和CYP3A4介导. 中國藥理學報(英文版). 2000, 21 (8): 747-752.
- ^ 唐汝愚,姚丹帆,周文正. 国产新抗疟药——氯苯三嗪抗疟作用的研究. 中華醫學雜誌. 1957, 43 (4): 286-291.
- ^ Srivastava IK, Vaidya AB. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrobial Agents and Chemotherapy. June 1999, 43 (6): 1334–9. PMC 89274 . PMID 10348748. doi:10.1128/AAC.43.6.1334.
- ^ Watkins WM, Sixsmith DG, Chulay JD. The activity of proguanil and its metabolites, cycloguanil and p-chlorophenylbiguanide, against Plasmodium falciparum in vitro (Free full text). Annals of Tropical Medicine and Parasitology. June 1984, 78 (3): 273–8 [2024-09-16]. PMID 6385887. doi:10.1080/00034983.1984.11811816. (原始內容存檔於2010-03-07).
- ^ Thapar MM, Gupta S, Spindler C, Wernsdorfer WH, Björkman A. Pharmacodynamic interactions among atovaquone, proguanil and cycloguanil against Plasmodium falciparum in vitro. Transactions of the Royal Society of Tropical Medicine and Hygiene. May 2003, 97 (3): 331–7. PMID 15228254. doi:10.1016/S0035-9203(03)90162-3 .
- ^ Walzer PD, Foy J, Steele P, White M. Synergistic combinations of Ro 11-8958 and other dihydrofolate reductase inhibitors with sulfamethoxazole and dapsone for therapy of experimental pneumocystosis. Antimicrobial Agents and Chemotherapy. July 1993, 37 (7): 1436–43. PMC 187990 . PMID 8363372. doi:10.1128/AAC.37.7.1436.
- ^ Modest, Edward J. (1956). "Chemical and Biological Studies on 1,2-Dihydro-s-triazines. II. Three-Component Synthesis". The Journal of Organic Chemistry 21 (1): 1–13. doi:10.1021/jo01107a001.
- ^ Modest, Edward J.; Levine, Philip. (1956). "Chemical and Biological Studies on 1,2-Dihydro-s-triazines. III. Two-Component Synthesis". The Journal of Organic Chemistry. 21(1): 14–20. doi:10.1021/jo01107a002.
- ^ Carrington, H. C.; Crowther, A. F.; Stacey, G. J. (1954). "Synthetic antimalarials. Part XLIX. The structure and synthesis of the dihydrotriazine metabolite of proguanil". Journal of the Chemical Society (Resumed): 1017. doi:10.1039/jr9540001017.
- ^ Modest, Edward J.; Foley, George E.; Pechet, Maurice M.; Farber, Sidney (1952). "A SERIES OF NEW, BIOLOGICALLY SIGNIFICANT DIHYDROTRIAZINES". Journal of the American Chemical Society. 74 (3): 855–856. doi:10.1021/ja01123a532.
- ^ Loo, Ti Li (1954). "1-p-Chlorophenyl-2,4-diamino-6,6-dimethyl-1,6-dihydro-1,3,5- triazine". Journal of the American Chemical Society. 76 (20): 5096–5099. doi:10.1021/ja01649a026.
- ^ Edward J. Modest, 美國專利第2,900,385號 (1959 to Children s Cancer Research Foundation).
- ^ Donald F. Worth, 美國專利第3,074,947號 (1963 to Parke).