AM大麻素列表
维基媒体列表条目
美國麻薩諸塞州東北大學亞歷山德羅斯·馬克里揚尼斯(Alexandros Makriyannis)的研究小組合成了許多大麻素,統稱為AM大麻素,其中一些是:
| 名稱 | 類別 | 分子式 | Ki/nM (CB1) | Ki/nM (CB2) | 選擇性 | 結構 | ref |
|---|---|---|---|---|---|---|---|
| AM-087 | 二苯並吡喃 | C23H33BrO2 | 0.43 |  |
|||
| AM-251 | 吡唑衍生物 | C22H21Cl2IN4O | 7.5 |  |
[1] | ||
| AM-356 | C23H39NO2 | 17.9 | 868 |  |
[2] | ||
| AM-404 | C26H37NO2 |  |
|||||
| AM-411 | 二苯並吡喃 | C26H34O2 | 6.8 | 52.0 |  |
||
| AM-630 | 苯甲醯基吲哚 | C23H25IN2O3 | 32.1 | CB2(165×) |  |
||
| AM-678 | 萘甲醯基吲哚 | C24H23NO | 9.00±5.00 | 2.94±2.65 | CB2 |  |
|
| AM-679 | 苯甲醯基吲哚 | C20H20INO | 13.5 | 49.5 | CB1 | 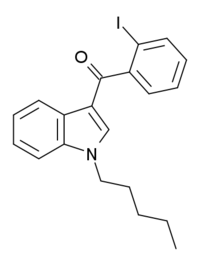 |
|
| AM-694 | 苯甲醯基吲哚 | C20H19FINO | 0.08 | 1.44 | CB1(18×) |  |
[3] |
| AM-855 | C26H38O2 | 22.3 | 58.6 | CB1 |  |
||
| AM-905 | C23H34O3 | 1.2 | 5.3 | CB1 |  |
||
| AM-906 | C23H34O3 | 0.8 | 9.5 | CB1 |  |
||
| AM-919 | C27H44O4 | 2.2 | 3.4 | CB1 |  |
||
| AM-938 | C27H40O4 | 1.2 | 0.3 | CB2(4×) |  |
||
| AM-1220 | 萘甲醯基吲哚 | C26H26N2O | 3.88 | 73.4 | CB1 (19×) |  |
[4][5] |
| 名稱 | 類別 | 分子式 | 結構 | CAS號 |
|---|---|---|---|---|
| AMG-1 | 二苯並吡喃 | C23H30O2 |  |
205746-46-9 |
| AMG-3 | 二苯並吡喃 | C25H36O2S2 |  |
179044-94-1 |
| AMG-36 | 二苯並吡喃 | C27H40O2 |  |
579444-43-2 |
| AMG-41 | 二苯並吡喃 | C25H36O2 |  |
499237-35-3 |
參見
編輯參考文獻
編輯- ^ Lan, Ruoxi; Lu, Qian; Fan, Pusheng; Gatley, John; Volkow, Nora D.; Fernando, Susanthi R.; Pertwee, Roger; Makriyannis, Alexandros. Design and synthesis of the CB1 selective cannabinoid antagonist AM281: A potential human SPECT ligand. AAPS PharmSci. 1999, 1 (2): 39–45. PMC 2761119 . PMID 11741201. doi:10.1208/ps010204.
- ^ Selwood, D. The Cannabinoid Receptors. Edited by Patricia H. Reggio. ChemMedChem. 2009, 4: 1949. doi:10.1002/cmdc.200900286.
- ^ Template:Ref patent2
- ^ D'ambra, T. C-Attached aminoalkylindoles: potent cannabinoid mimetics. Bioorganic & Medicinal Chemistry Letters. 1996, 6: 17–22. doi:10.1016/0960-894X(95)00560-G.
- ^ Willis, P. G.; Pavlova, O. A.; Chefer, S. I.; Vaupel, D. B.; Mukhin, A. G.; Horti, A. G. Synthesis and Structure−Activity Relationship of a Novel Series of Aminoalkylindoles with Potential for Imaging the Neuronal Cannabinoid Receptor by Positron Emission Tomography. Journal of Medicinal Chemistry. 2005, 48 (18): 5813–22. PMID 16134948. doi:10.1021/jm0502743.