环氧环己烷
化合物
环氧环己烷(英語:Cyclohexene oxide,Epoxycyclohexane)是一种环烷烃环氧化合物。其可作为单体经阳离子聚合成具有热塑性的聚环氧环己烷。
| 环氧环己烷 | |
|---|---|
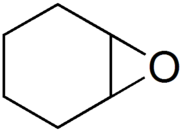
| |
| IUPAC名 7-Oxabicyclo[4.1.0]heptane | |
| 别名 | 氧化环己烯 |
| 识别 | |
| CAS号 | 286-20-4 |
| PubChem | 9246 |
| ChemSpider | 8890 |
| SMILES |
|
| 性质 | |
| 化学式 | C6H10O |
| 摩尔质量 | 98.14 g·mol−1 |
| 外观 | 无色液体[1] |
| 密度 | 0.97 g·cm−3[1] |
| 熔点 | ca. -40 °C[1] |
| 沸点 | ca. 130 °C[1] |
| 溶解性(水) | 部分溶解[1] |
| 蒸氣壓 | 12 mbar (at 20 °C)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
合成
编辑环氧环己烷可由环己烯经环氧化反应得到。反应体系可以与过氧酸进行均相反应得到[2],也可以利用催化剂(如银与氧气反应)进行非均相反应得到[3][4][5]。
工业生产环氧环己烷更倾向于采用非均相催化反应,因为这样有更好的原子经济性,而且产物容易分类,催化剂可回收重复利用。采用的过氧酸常为过氧化氢[6]。近年来也发现了采用固定化的金属卟啉络合物催化氧化有很高的效率[7][8]。
在实验中,常用环己烯与单过氧邻苯二甲酸镁(MMPP)的水-异丙醇溶液室温下反应得到环氧环己烷,且反应产率可达到85%[9]。
性质和反应
编辑环氧环己烷的晶体结构已通过分析方法得到广泛研究。环氧环己烷可在溶液中经固体酸催化剂的催化下聚合形成聚环氧环己烷[10]。
参考文献
编辑- ^ 1.0 1.1 1.2 1.3 1.4 1.5 Record of Epoxycyclohexane in the GESTIS Substance Database from the IFA, accessed on 1 February 2014
- ^ M. Quenard; V. Bonmarin; G. Gelbard. Epoxidation of olefins by hydrogen peroxide catalyzed by phosphonotungstic complexes. Tetrahedron Letters. 1987, 28 (20): 2237–2238. doi:10.1016/S0040-4039(00)96089-1.
- ^ Ha Q. Pham; Maurice J. Marks, Epoxy Resins, Ullmann's Encyclopedia of Industrial Chemistry, 2005, ISBN 3527306730, doi:10.1002/14356007.a09_547.pub2 (德语)
- ^ Siegfried Rebsdat; Dieter Mayer, Ethylene Oxide, Ullmann's Encyclopedia of Industrial Chemistry, 2001, ISBN 3527306730, doi:10.1002/14356007.a10_117 (德语)
- ^ Morazzoni, Franca; Canevali, Carmen; d'Aprile, Fiorenza; Bianchi, Claudia L.; Tempesti, Ezio; Giuffrè, Luigi; Airoldi, Giuseppe. Spectroscopic investigation of the molybdenum active sites on MoVI heterogeneous catalysts for alkene epoxidation. Journal of the Chemical Society, Faraday Transactions. 1995, 91 (21): 3969–3974. doi:10.1039/FT9959103969.
- ^ Ambili, V K; Dr.Sugunan, S, Faculty of Sciences , 编, Studies on Catalysis by Ordered Mesoporous SBA-15 Materials Modified with Transition Metals, Cochin University of Science and Technology, April 2011 [2014-07-27] (德语)
- ^ Costa, Andréia A. Ghesti; Grace F. de Macedo; Julio L. Braga; Valdeilson S. Santos; Marcello M. Dias; José A. Dias; Sílvia C.L. Immobilization of Fe, Mn and Co tetraphenylporphyrin complexes in MCM-41 and their catalytic activity in cyclohexene oxidation reaction by hydrogen peroxide. Journal of Molecular Catalysis A: Chemical. 2008, 282 (1–2): 149–157. doi:10.1016/j.molcata.2007.12.024.
- ^ Xian-Tai Zhou; Hong-Bing Ji; Jian-Chang Xu; Li-Xia Pei; Le-Fu Wang; Xing-Dong Yao. Enzymatic-like mediated olefins epoxidation by molecular oxygen under mild conditions. Tetrahedron Letters. 2007, 48 (15): 2691–2695. doi:10.1016/j.tetlet.2007.02.066.
- ^ Brougham, Paul; Cooper, Mark S.; Cummerson, David A.; Heaney, Harry; Thompson, Nicola. Oxidation Reactions Using Magnesium Monoperphthalate: A Comparison with m-Chloroperoxybenzoic Acid. Synthesis. 1987, 1987 (11): 1015–1017 [2020-07-31]. doi:10.1055/s-1987-28153.
- ^ Ahmed Yahiaoui; Mohammed Belbachir; Jeanne Claude Soutif; Laurent Fontaine. Synthesis and structural analyses of poly(1,2-cyclohexene oxide) over solid acid catalyst. Materials Letters. 2005, 59 (7): 759–767. doi:10.1016/j.matlet.2004.11.017.
| 这是一篇與化学相關的小作品。您可以通过编辑或修订扩充其内容。 |