苯丙氨酸羟化酶
苯丙氨酸羟化酶 (英語:Phenylalanine hydroxylase,简称PheOH,或者PheH、PAH) (EC 1.14.16.1) 是一种将苯丙氨酸侧链上的苯环羟基化为酪氨酸的羟化酶。 PheOH是使用四氢生物蝶呤(BH4,蝶啶类辅因子)和亚铁离子(Fe2+)作为辅酶的单加氧酶类蛋白质三种中的一种。在反应中,一个氧分子的两个氧原子被分别加入到BH4和苯丙氨酸中。[1]
| 苯丙氨酸羟化酶 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
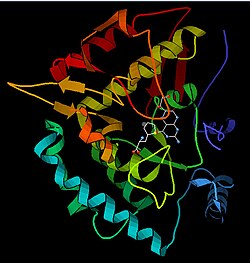 苯丙氨酸羟化酶的三维结构预测 | |||||||||||||
| |||||||||||||
| 标识 | |||||||||||||
| 代号 | PAH; PH; PKU; PKU1 | ||||||||||||
| 扩展标识 | 遗传学:612349 鼠基因:97473 同源基因:234 ChEMBL: 3076 GeneCards: PAH Gene | ||||||||||||
| EC編號 | 1.14.16.1 | ||||||||||||
| RNA表达模式 | |||||||||||||
 | |||||||||||||
 | |||||||||||||
| 更多表达数据 | |||||||||||||
| 直系同源体 | |||||||||||||
| 物种 | 人类 | 小鼠 | |||||||||||
| Entrez | 5053 | 18478 | |||||||||||
| Ensembl | ENSG00000171759 | ENSMUSG00000020051 | |||||||||||
| UniProt | P00439 | P16331 | |||||||||||
| mRNA序列 | NM_000277 | NM_008777 | |||||||||||
| 蛋白序列 | NP_000268 | NP_032803 | |||||||||||
| 基因位置 |
Chr 12: 103.23 – 103.35 Mb |
Chr 10: 87.52 – 87.58 Mb | |||||||||||
| PubMed查询 | [1] | [2] | |||||||||||
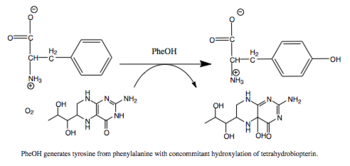 |
苯丙氨酸羟化酶是体内过量苯丙氨酸分解代谢的限速酶。Seymour Kaufman关于苯丙氨酸羟化酶的研究使一种新的生物辅酶四氢生物蝶呤被发现。[2] 此酶受到人类健康方面的关注是因为编码该酶的PAH基因如果发生突变,会造成严重的代谢紊乱即苯丙酮尿症
酶反应机制
编辑反应认为是按照以下步骤进行:
- 形成 Fe(II)-O-O-BH4 桥.
- O-O键异裂产生铁氧羟化中间体 Fe(IV)=O.
- attack on Fe(IV)=O to hydroxylate phenylalanine substrate to tyrosine.[3]
酶调控
编辑结构
编辑51.9千道尔顿的PheOH蛋白质分子单体可以分为三个结构域: N端结构域 (氨基酸残基1-117), 催化结构域 (残基118-427), 以及负责相同单体聚合的C端结构域 (残基428-453) 。
生物学功能
编辑PheOH是苯丙氨酸完全分解代谢为二氧化碳和水这一过程的关键酶,催化限速步骤。[5]
有关酶
编辑苯丙氨酸羟化酶与以下两种酶密切相关:
这三种酶是同源的,由共同的祖先古羟化酶进化而来。
参考文献
编辑- ^ Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 1999, 68: 355–81. PMID 10872454. doi:10.1146/annurev.biochem.68.1.355.
- ^ KAUFMAN S. A new cofactor required for the enzymatic conversion of phenylalanine to tyrosine. J. Biol. Chem. February 1958, 230 (2): 931–9. PMID 13525410.
- ^ Fitzpatrick PF. Mechanism of aromatic amino acid hydroxylation. Biochemistry. December 2003, 42 (48): 14083–91. PMC 1635487 . PMID 14640675. doi:10.1021/bi035656u.
- ^ T. Selwood and E. K. Jaffe. Dynamic dissociating homo-oligomers and the control of protein function.. Arch. Biochem. Biophys. 2011, 519 (2): 131–43 [2013-04-28]. PMC 3298769 . PMID 22182754. doi:10.1016/j.abb.2011.11.020. (原始内容存档于2007-05-12).
- ^ Kaufman S. A model of human phenylalanine metabolism in normal subjects and in phenylketonuric patients. Proc. Natl. Acad. Sci. U.S.A. March 1999, 96 (6): 3160–4. PMC 15912 . PMID 10077654. doi:10.1073/pnas.96.6.3160.
扩展阅读
编辑- Eisensmith RC, Woo SL. Molecular basis of phenylketonuria and related hyperphenylalaninemias: mutations and polymorphisms in the human phenylalanine hydroxylase gene.. Hum. Mutat. 1993, 1 (1): 13–23. PMID 1301187. doi:10.1002/humu.1380010104.
- Konecki DS, Lichter-Konecki U. The phenylketonuria locus: current knowledge about alleles and mutations of the phenylalanine hydroxylase gene in various populations.. Hum. Genet. 1991, 87 (4): 377–88. PMID 1679029. doi:10.1007/BF00197152.
- Cotton RG. Heterogeneity of phenylketonuria at the clinical, protein and DNA levels.. J. Inherit. Metab. Dis. 1991, 13 (5): 739–50. PMID 2246858. doi:10.1007/BF01799577.
- Erlandsen H, Fusetti F, Martinez A; et al. Crystal structure of the catalytic domain of human phenylalanine hydroxylase reveals the structural basis for phenylketonuria.. Nat. Struct. Biol. 1998, 4 (12): 995–1000. PMID 9406548. doi:10.1038/nsb1297-995.
- Waters PJ, Parniak MA, Nowacki P, Scriver CR. In vitro expression analysis of mutations in phenylalanine hydroxylase: linking genotype to phenotype and structure to function.. Hum. Mutat. 1998, 11 (1): 4–17. PMID 9450897. doi:10.1002/(SICI)1098-1004(1998)11:13.0.CO;2-L.
- Waters PJ. How PAH gene mutations cause hyper-phenylalaninemia and why mechanism matters: insights from in vitro expression.. Hum. Mutat. 2003, 21 (4): 357–69. PMID 12655545. doi:10.1002/humu.10197.