丙帕他莫
化合物
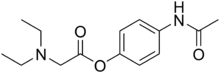
丙帕他莫是扑热息痛的前药,由扑热息痛和羧酸二乙基甘氨酸酯化形成。这具有使其更易溶于水的优点。它用于术后透过静脉注射护理无法以口服或直肠给药扑热息痛,并且禁用非甾体抗炎药(NSAID)的患者。丙帕他莫起效比口服扑热息痛快。 [3] 2克丙帕他莫相当于1 克扑热息痛的药效。 [4]
 | |
| 临床资料 | |
|---|---|
| AHFS/Drugs.com | 国际药品名称 |
| 给药途径 | IV[1][2] |
| ATC码 | |
| 药物动力学数据 | |
| 生物半衰期 | 2.4小时 [1] |
| 排泄途径 | 经由肾脏 |
| 识别信息 | |
| |
| CAS号 | 66532-85-2 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.060.336 |
| 化学信息 | |
| 化学式 | C14H20N2O3 |
| 摩尔质量 | 264.33 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
相关条目
编辑参考资料
编辑- ^ 1.0 1.1 Bannwarth B, Netter P, Lapicque F, Gillet P, Péré P, Boccard E, Royer RJ, Gaucher A. Plasma and cerebrospinal fluid concentrations of paracetamol after a single intravenous dose of propacetamol. British Journal of Clinical Pharmacology. 1992, 34 (1): 79–81. PMC 1381380 . PMID 1633071. doi:10.1111/j.1365-2125.1992.tb04112.x.
- ^ Binhas M, Decailliot F, Rezaiguia-Delclaux S, Suen P, Dumerat M, François V, Combes X, Duvaldestin P. Comparative effect of intraoperative propacetamol versus placebo on morphine consumption after elective reduction mammoplasty under remifentanil-based anesthesia: a randomized control trial [ISRCTN71723173]. BMC Anesthesiology. 2004, 4 (1): 6. PMC 520811 . PMID 15367329. doi:10.1186/1471-2253-4-6.
- ^ Onset of acetaminophen analgesia: comparison of oral and intravenous routes after third molar surgery. British Journal of Anaesthesiology. 2005, 94 (5): 642–648. PMID 15790675. doi:10.1093/bja/aei109.
- ^ Bioequivalence study comparing a new paracetamol solution for injection and propacetamol after single intravenous infusion in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics. 2004, 42 (1): 50–57. PMID 14756388. doi:10.5414/cpp42050.