3C樣蛋白酶
3C樣蛋白酶(3CLpro,英語:3C-like protease)或稱主蛋白酶(Mpro),前稱C30內肽酶或3-糜蛋白酶樣蛋白酶,[2]是在冠狀病毒中發現的主要蛋白酶。它在11個保守位點切割冠狀病毒多聚蛋白。它是一種半胱氨酸蛋白酶,也是蛋白酶PA家族的成員。它在其活性位點具有半胱氨酸-組氨酸催化二聯體,並切Gln-(Ser/Ala/Gly)肽鍵。
| SARS冠狀病毒主蛋白酶 | |||||||
|---|---|---|---|---|---|---|---|
| |||||||
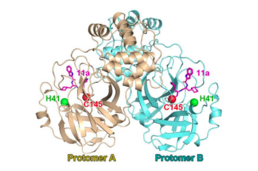
| SARS冠狀病毒主蛋白酶二聚體與催化二聯體(H41;C145)與共價肽模擬蛋白酶抑制劑(「11a」,洋紅色)複合。從 PDB 6LZE.[1] | |||||||
| |||||||
| 識別碼 | |||||||
| EC編號 | 3.4.22.69 | ||||||
| 數據庫 | |||||||
| IntEnz | IntEnz瀏覽 | ||||||
| BRENDA | BRENDA入口 | ||||||
| ExPASy | NiceZyme瀏覽 | ||||||
| KEGG | KEGG入口 | ||||||
| MetaCyc | 代謝路徑 | ||||||
| PRIAM | 概述 | ||||||
| PDB | RCSB PDB PDBj PDBe PDBsum | ||||||
| |||||||
| C30肽酶、冠狀病毒內肽酶 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 鑑定 | |||||||||
| 標誌 | Peptidase_C30 | ||||||||
| Pfam | PF05409(舊版) | ||||||||
| InterPro | IPR008740 | ||||||||
| PROSITE | PS51442 | ||||||||
| MEROPS | C30 | ||||||||
| SCOP | d1q2wb1 / SUPFAM | ||||||||
| |||||||||
酶學委員會將這個家族稱為SARS冠狀病毒主蛋白酶(Mpro; EC 3.4.22.69)。3C樣蛋白酶對應了冠狀病毒非結構蛋白5(nsp5)。通用名稱中的「3C」是指3C蛋白酶(3Cpro),它是一種存在於微小核糖核酸病毒中的同源蛋白酶。
功能
編輯3C樣蛋白酶能夠催化裂解P1位穀氨酰胺和P1'位小氨基酸(絲氨酸、丙氨酸或甘氨酸)之間的肽鍵。例如,SARS 冠狀病毒 3CLpro可以自我切割以下肽:[3][4][5]
蛋白酶在冠狀病毒複製酶多蛋白的加工中很重要(UniProt P0C6U8)。它是冠狀病毒中的主要蛋白酶,對應於非結構蛋白5 (nsp5)。[6]它在11個保守位點切割冠狀病毒多蛋白。 3C樣蛋白酶在其活性位點具有半胱氨酸-組氨酸催化二聯體。[4]半胱氨酸的硫作為親核試劑,組氨酸的咪唑環作為一般鹼基。[7]
| 位置 | 基材偏好 |
|---|---|
| P5 | 無強烈的偏好 |
| P4 | 小的疏水殘基 |
| P3 | 帶正電荷的殘基 |
| P2 | 高疏水性和無β-支鏈 |
| P1 | 穀氨酰胺 |
| P1' | 少量殘留物 |
| P2' | 少量殘留物 |
| P3' | 無強烈的偏好 |
命名法
編輯EC提供的替代名稱包括3CLpro、3C樣蛋白酶、冠狀病毒3C樣蛋白酶、Mpro、SARS 3C樣蛋白酶、SARS冠狀病毒3CL蛋白酶、SARS冠狀病毒主肽酶、SARS冠狀病毒主蛋白酶、SARS-CoV 3CLpro酶、SARS-CoV主蛋白酶、SARS-CoV Mpro和嚴重急性呼吸綜合徵冠狀病毒主蛋白酶。
作為治療靶點
編輯]
3C蛋白酶是冠狀病毒感染的潛在藥物靶標,因為它在處理從病毒RNA翻譯的多聚蛋白中起重要作用。[11][12]未配位的3C蛋白酶及其與α-酮酰胺抑制劑的複合物的X射線結構[13]為設計用於治療2型新冠病毒感染的α-酮酰胺抑制劑提供了基礎。[14][15][16][17][18]
許多針對3CLpro和同源3Cpro的蛋白酶抑制劑正在開發中,包括3CLpro-1、GC376、蘆平曲韋、盧夫特韋、奈瑪特韋和AG7404。[19][20][21][22][1]靜脈注射前體藥物lufotrelvir於2020年9月進入臨床試驗。[23]口服活性的後續藥物奈瑪特韋作為與利托那韋的組合藥物處於 II/III 期臨床試驗中,並於2021年11月公布了結果,包括在COVID-19症狀發作後的三天內給予藥物治療可以減少89%的住院率。[24]一項包含2.35億個分子的超大型虛擬篩選活動能夠識別出一種針對幾種冠狀病毒主要蛋白酶的新型廣譜抑制劑。[25]
2022年5月25日,英科智能通過人工智能來探索使用3C樣蛋白酶抑制劑治療COVID-19。臨床前候選藥物具有由人工智能設計的專門結構,被提名用於靶向3C樣蛋白酶。它充當抑制劑並與2型新冠病毒和MERS病毒的3C樣蛋白酶結合,使其失效並抑制病毒複製。臨床前候選藥物用於通過防止冠狀病毒複製來治療已經感染的人。[26]
其它3C(樣)蛋白酶
編輯3C樣蛋白酶廣泛存在於(+)ssRNA病毒中。它們都是半胱氨酸蛋白酶,具有糜蛋白酶樣摺疊(PA族),使用催化二聯體或三聯體。它們在底物特異性和抑制劑有效性方面有一些普遍的相似之處。它們按序列相似性分為亞家族,對應於它們所在的病毒家族:[27]
參見
編輯參考文獻
編輯- ^ 1.0 1.1 1.2 Dai W, Zhang B, Jiang XM, Su H, Li J, Zhao Y, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. June 2020, 368 (6497): 1331–1335. Bibcode:2020Sci...368.1331D. PMC 7179937 . PMID 32321856. doi:10.1126/science.abb4489 .
- ^ Ahmad, Bilal; Batool, Maria; Ain, Qurat ul; Kim, Moon Suk; Choi, Sangdun. Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations. International Journal of Molecular Sciences. 2021-08-24, 22 (17) [2022-09-16]. ISSN 1422-0067. PMC 8430524 . PMID 34502033. doi:10.3390/ijms22179124. (原始內容存檔於2021-10-06).
- ^ Goetz DH, Choe Y, Hansell E, Chen YT, McDowell M, Jonsson CB, Roush WR, McKerrow J, Craik CS. Substrate specificity profiling and identification of a new class of inhibitor for the major protease of the SARS coronavirus. Biochemistry. July 2007, 46 (30): 8744–52. PMID 17605471. doi:10.1021/bi0621415.
- ^ 4.0 4.1 Fan K, Wei P, Feng Q, Chen S, Huang C, Ma L, Lai B, Pei J, Liu Y, Chen J, Lai L. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. The Journal of Biological Chemistry. January 2004, 279 (3): 1637–42. PMC 7980035 . PMID 14561748. doi:10.1074/jbc.m310875200 .
- ^ Akaji K, Konno H, Onozuka M, Makino A, Saito H, Nosaka K. Evaluation of peptide-aldehyde inhibitors using R188I mutant of SARS 3CL protease as a proteolysis-resistant mutant. Bioorganic & Medicinal Chemistry. November 2008, 16 (21): 9400–8. PMC 7126698 . PMID 18845442. doi:10.1016/j.bmc.2008.09.057.
- ^ Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Maier HJ, Bickerton E, Britton P (編). Coronaviruses. Methods in Molecular Biology 1282. Springer. 2015: 1–23. ISBN 978-1-4939-2438-7. PMC 4369385 . PMID 25720466. doi:10.1007/978-1-4939-2438-7_1.
See section: Virion Structure.
- ^ Ryu YB, Park SJ, Kim YM, Lee JY, Seo WD, Chang JS, et al. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorganic & Medicinal Chemistry Letters. March 2010, 20 (6): 1873–6. ISSN 0960-894X. PMC 7127101 . PMID 20167482. doi:10.1016/j.bmcl.2010.01.152.
- ^ Chuck CP, Chow HF, Wan DC, Wong KB. Profiling of substrate specificities of 3C-like proteases from group 1, 2a, 2b, and 3 coronaviruses. PLoS One. 2011, 6 (11): e27228. PMC 3206940 . PMID 22073294. doi:10.1371/journal.pone.0027228.
- ^ Vandyck K, Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr Opin Virol. August 2021, 49: 36–40. PMC 8075814 . PMID 34029993. doi:10.1016/j.coviro.2021.04.006.
- ^ Pfizer begins dosing in Phase II/III trial of antiviral drug for Covid-19.. Clinical Trials Arena. 2 September 2021.
- ^ Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS Journal. July 2014, 281 (18): 4085–4096. PMID 25039866. doi:10.1111/febs.12936.
- ^ Ullrich S, Nitsche C. The SARS-CoV-2 main protease as a drug target. Bioorganic & Medicinal Chemistry Letters. July 2020, 30 (17): 127377. PMID 32738988. doi:10.1016/j.bmcl.2020.127377.
- ^ Ocain TD, Rich DH. alpha-Keto amide inhibitors of aminopeptidases. Journal of Medicinal Chemistry. February 1992, 35 (3): 451–6. PMID 1738140. doi:10.1021/jm00081a005.
- ^ Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. June 2003, 300 (5626): 1763–7. Bibcode:2003Sci...300.1763A. PMID 12746549. doi:10.1126/science.1085658 .
- ^ Pacifico S, Ferretti V, Albanese V, Fantinati A, Gallerani E, Nicoli F, et al. Synthesis and Biological Activity of Peptide α-Ketoamide Derivatives as Proteasome Inhibitors. ACS Medicinal Chemistry Letters. July 2019, 10 (7): 1086–1092. PMC 6627721 . PMID 31312413. doi:10.1021/acsmedchemlett.9b00233.
- ^ Kusov Y, Tan J, Alvarez E, Enjuanes L, Hilgenfeld R. A G-quadruplex-binding macrodomain within the "SARS-unique domain" is essential for the activity of the SARS-coronavirus replication-transcription complex. Virology. October 2015, 484: 313–22. PMC 4567502 . PMID 26149721. doi:10.1016/j.virol.2015.06.016.
- ^ Zhang L, Lin D, Kusov Y, Nian Y, Ma Q, Wang J, et al. α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment. Journal of Medicinal Chemistry. February 2020, 63 (9): 4562–4578. PMC 7098070 . PMID 32045235. doi:10.1021/acs.jmedchem.9b01828.
- ^ Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. March 2020, 368 (6489): 409–412. Bibcode:2020Sci...368..409Z. PMC 7164518 . PMID 32198291. doi:10.1126/science.abb3405 .
- ^ Tian D, Liu Y, Liang C, Xin L, Xie X, Zhang D, Wan M, Li H, Fu X, Liu H, Cao W. An update review of emerging small-molecule therapeutic options for COVID-19. Biomedicine & Pharmacotherapy. May 2021, 137: 111313. PMC 7857046 . PMID 33556871. doi:10.1016/j.biopha.2021.111313.
- ^ Morse JS, Lalonde T, Xu S, Liu WR. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. ChemBioChem. March 2020, 21 (5): 730–738. PMC 7162020 . PMID 32022370. doi:10.1002/cbic.202000047.
- ^ Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Central Science. March 2020, 6 (3): 315–331. PMC 7094090 . PMID 32226821. doi:10.1021/acscentsci.0c00272 .
- ^ Ramajayam R, Tan KP, Liang PH. Recent development of 3C and 3CL protease inhibitors for anti-coronavirus and anti-picornavirus drug discovery. Biochemical Society Transactions. October 2011, 39 (5): 1371–5. PMID 21936817. doi:10.1042/BST0391371 .
- ^ First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19.. Clinical Trials. 24 June 2021 [3 July 2021]. (原始內容存檔於2021-11-05).
- ^ Pfizer's Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk Of Hospitalization Or Death By 89% In Interim Analysis Of Phase 2/3 EPIC-HR Study. Pfizer Inc. 5 November 2021 [2022-09-16]. (原始內容存檔於2021-11-16).
- ^ Luttens A, Gullberg H, Abdurakhmanov E, Vo DD, Akaberi D, Talibov VO, et al. Ultralarge Virtual Screening Identifies SARS-CoV-2 Main Protease Inhibitors with Broad-Spectrum Activity against Coronaviruses. J Am Chem Soc. February 2022, 144 (7): 2905–2920. ISSN 0002-7863. PMC 8848513 . PMID 35142215. doi:10.1021/jacs.1c08402.
- ^ AI-designed COVID-19 drug nominated for preclinical trials. www.theregister.com. [2022-05-26]. (原始內容存檔於2022-12-01) (英語).
- ^ Kim Y, Lovell S, Tiew KC, Mandadapu SR, Alliston KR, Battaile KP, et al. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. Journal of Virology. November 2012, 86 (21): 11754–62. PMC 3486288 . PMID 22915796. doi:10.1128/JVI.01348-12.
- ^ Ziebuhr J, Bayer S, Cowley JA, Gorbalenya AE. The 3C-like proteinase of an invertebrate nidovirus links coronavirus and potyvirus homologs. Journal of Virology. January 2003, 77 (2): 1415–26. PMC 140795 . PMID 12502857. doi:10.1128/jvi.77.2.1415-1426.2003 .