α-乙酰-γ-丁內酯
化合物
ɑ-乙酰-γ-丁內酯(ABL)是γ-丁內酯的衍生物,可用於有機合成,也用於鑑定一級胺。[2]
| Α-乙酰-γ-丁內酯 | |
|---|---|
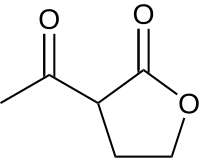
| |
| IUPAC名 3-acetyloxolan-2-one | |
| 英文名 | 2-Acetylbutyrolactone |
| 別名 | 2-乙酰-γ-丁內酯 |
| 識別 | |
| 縮寫 | ABL |
| CAS編號 | 517-23-7 |
| PubChem | 10601 |
| ChemSpider | 10156 |
| SMILES |
|
| InChI |
|
| EINECS | 208-235-2 |
| ChEBI | 179633 |
| MeSH | 2-acetylbutyrolactone |
| 性質 | |
| 化學式 | C6H8O3 |
| 摩爾質量 | 128.13 g·mol−1 |
| 外觀 | 無色液體 |
| 密度 | 1.19 g/cm3 [1] |
| 沸點 | 107-108 °C(380-381 K)(7 hPa[1]) |
| 溶解性 | 可溶於DMF[2]和甲醇[3] |
| 危險性 | |
GHS危險性符號 [4] [4]
| |
| GHS提示詞 | Warning |
| H-術語 | H315, H319, H335 |
| P-術語 | P261, P271, P280, P302+352, P304+340, P305+351+338, P321, P362+364, P403+233, P405, P501 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
製備
編輯用處
編輯熒光檢驗
編輯ɑ-乙酰-γ-丁內酯本身的熒光很微弱,但它的衍生物照到紫外線時會發出很強的熒光。[3][6]ɑ-乙酰-γ-丁內酯的羰基會和胺反應生成希夫鹼,因此經常用於在有機合成中確認合成出了胺。[3]ɑ-乙酰-γ-丁內酯也可以和芳香胺發生雅普-克林格曼反應,生成熒光分子。[6]
藥物的前體
編輯參考文獻
編輯- ^ 1.0 1.1 α-Acetylbutyrolactone Safety Data Sheet. SigmaAldrich. January 20, 2020 [March 30, 2022]. (原始內容存檔於2022-03-31).
- ^ 2.0 2.1 Sabry, Suzy M. Application of 2-acetylbutyrolactone to spectrofluorimetry: Fluorescence properties of Schiff bases derived from 2-acetylbutyrolactone and spectrofluorimetric determination of primary amine-containing compounds. Journal of Pharmaceutical and Biomedical Analysis. 2006, 40 (5): 1057–1067 [2022-06-12]. PMID 16256289. doi:10.1016/j.jpba.2005.08.036. (原始內容存檔於2018-06-27) (英語).
- ^ 3.0 3.1 3.2 Sabry, S M. Application of 2-acetylbutyrolactone to spectrofluorimetry: Fluorescence properties of Schiff bases derived from 2-acetylbutyrolactone and spectrofluorimetric determination of primary amine-containing compounds. Journal of Pharmaceutical and Biomedical Analysis. 2006, 40 (5): 1057–1067. PMID 16256289. doi:10.1016/j.jpba.2005.08.036.
- ^ 2-Acetylbutyrolactone. pubchem.ncbi.nlm.nih.gov. [31 March 2022]. (原始內容存檔於2022-08-29) (英語).
- ^ Koehler, Guenther. Method for Preparing 2-Acetyl-y-Butyrolactone. United States Patent. August 4, 1998 [March 30, 2022]. (原始內容存檔於2022-03-31).
- ^ 6.0 6.1 Sabry, S M. Enhanced Spectrophotometry of Sulfonamides with Novel 2‐Acetylbutyrolactone Derivatives. Analytical Letters. 2006, 39 (13): 2591–2615. S2CID 93950011. doi:10.1080/00032710600824748.
- ^ Unnikrishnan, P,A. Syntheses of epi-β-Santalene, β-Santalene and an Isomer of β-Santalene with 4-Methyl-4-pentenyl Side Chain. Synthetic Communications. 1992, 22 (22): 3159–3168. doi:10.1080/00397919208021129.