二乙酸碘苯
化合物
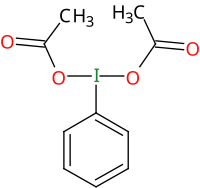
二乙酸碘苯(PIDA)是化学式C
6H
5I(OCOCH
3)
2的超价碘化合物,在有机化学中用作氧化剂。
| 二乙酸碘苯 | |
|---|---|
| |
| IUPAC名 Phenyl-λ3-iodanediyl diacetate | |
| 别名 | I,I-二乙酰氧基碘苯 PIDA |
| 识别 | |
| CAS号 | 3240-34-4 |
| PubChem | 76724 |
| ChemSpider | 69182 |
| SMILES |
|
| InChI |
|
| InChIKey | ZBIKORITPGTTGI-UHFFFAOYSA-N |
| 性质 | |
| 化学式 | C10H11IO4 |
| 摩尔质量 | 322.1 g·mol−1 |
| 外观 | 白色粉末 |
| 熔点 | 163-165 °C(436-438 K) |
| 溶解性(水) | 反应 |
| 溶解性 | 可溶于乙酸、乙腈和二氯甲烷 |
| 结构 | |
| 晶体结构 | 正交晶系 |
| 空间群 | Pnn2 |
| 晶格常数 | a = 15.693(3) Å, b = 8.477(2) Å, c = 8.762(2) Å |
| 分子构型 | T形分子构型 |
| 相关物质 | |
| 相关化学品 | 二(三氟乙酸)碘苯 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
制备
编辑结构
编辑PIDA分子是超价分子,它的碘原子形成了三个共价键。[7]这个分子呈T形分子构型,其中苯基占据水平位置,而乙酰氧基占据轴向位置,C-I-O键角低于90°。[8]二乙酸碘苯的晶体结构是正交晶系,空间群 Pnn2。[8][9]
反应
编辑PIDA可通过乙酰氧基的取代反应制备结构相似的试剂。举个例子,它在三氟乙酸中加热反应,可以得到二(三氟乙酸)碘苯(PIFA):[10][6]
产物PIFA则可以使酰胺在弱酸性环境[11],而不是以前需要的强碱性环境下发生霍夫曼降解反应。[12][13]N-保护的天冬酰胺可以用PIDA脱羰,这可用于合成β-氨基-L-丙氨酸衍生物。[14]
PIDA也可用于Suárez氧化反应中,这个反应可以把醇光解成环醚。[15][16][17]这个反应被用于数个全合成中,如(−)-majucin、(−)-Jiadifenoxolane A[18]和cephanolide A的全合成。[19]
参考资料
编辑- ^ Willgerodt, C. Zur Kenntniss aromatischer Jodidchloride, des Jodoso- und Jodobenzols. Chem. Ber. 1892, 25 (2): 3494–3502 [2022-08-24]. doi:10.1002/cber.189202502221. (原始内容存档于2022-05-24) (德语).
- ^ (1963) "Iodosobenzene Diacetate". Org. Synth. 43: 62; Coll. Vol. 5: 660.
- ^ 3.0 3.1 Moriarty, Robert M.; Chany, Calvin J.; Kosmeder, Jerome W.; Du Bois, Justin. (Diacetoxyiodo)benzene. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. 2001. ISBN 9780470842898. doi:10.1002/047084289x.rd005m.pub2.
- ^ Hossain, Md. Delwar; Kitamura, Tsugio. Unexpected, Drastic Effect of Triflic Acid on Oxidative Diacetoxylation of Iodoarenes by Sodium Perborate. A Facile and Efficient One-Pot Synthesis of (Diacetoxyiodo)arenes. J. Org. Chem. 2005, 70 (17): 6984–6986. PMID 16095332. doi:10.1021/jo050927n.
- ^ Hossain, Md. Delwar; Kitamura, Tsugio. New and Direct Approach to Hypervalent Iodine Compounds from Arenes and Iodine. Straightforward Synthesis of (Diacetoxyiodo)arenes and Diaryliodonium Salts Using Potassium μ-Peroxo-hexaoxodisulfate. Bull. Chem. Soc. Jpn. 2007, 80 (11): 2213–2219. doi:10.1246/bcsj.80.2213.
- ^ 6.0 6.1 Dohi, Toshifumi; Kita, Yasuyuki. Oxidizing Agents. Kaiho, Tatsuo (编). Iodine Chemistry and Applications. John Wiley & Sons. 2015: 277–302. ISBN 9781118878651.
- ^ Dohi, Toshifumi; Kita, Yasuyuki. Hypervalent Iodine. Kaiho, Tatsuo (编). Iodine Chemistry and Applications. John Wiley & Sons. 2015: 103–158. ISBN 9781118878651.
- ^ 8.0 8.1 Lee, Chow-Kong; Mak, Thomas C. W.; Li, Wai-Kee; Kirner, John F. Iodobenzene diacetate. Acta Crystallogr. B. 1977, 33 (5): 1620–1622. doi:10.1107/S0567740877006694 .
- ^ Alcock, Nathaniel W.; Countryman, Rachel M.; Esperås, Steinar; Sawyer, Jeffery F. Secondary bonding. Part 5. The crystal and molecular structures of phenyliodine(III) diacetate and bis(dichloroacetate). J. Chem. Soc., Dalton Trans. 1979, 1979 (5): 854–860. doi:10.1039/DT9790000854.
- ^ (1988) "Hofmann Rearrangement Under Mildly Acidic Conditions Using [I,I-Bis(Trifluoroacetoxy)Iodobenzene: Cyclobutylamine Hydrochloride from Cyclobutanecarboxamide]". Org. Synth. 66; Coll. Vol. 8: 132.
- ^ Aubé, Jeffrey; Fehl, Charlie; Liu, Ruzhang; McLeod, Michael C.; Motiwala, Hashim F. 6.15 Hofmann, Curtius, Schmidt, Lossen, and Related Reactions. Heteroatom Manipulations. Comprehensive Organic Synthesis II 6. 2014: 598–635. ISBN 9780080977430. doi:10.1016/B978-0-08-097742-3.00623-6.
- ^ Wallis, Everett S.; Lane, John F. The Hofmann Reaction. Org. React. 1946, 3 (7): 267–306. doi:10.1002/0471264180.or003.07.
- ^ Surrey, Alexander R. Hofmann Reaction. Name Reactions in Organic Chemistry 2nd. Academic Press. 1961: 134–136. ISBN 9781483258683.
- ^ Zhang, Lin-hua; Kauffman, Goss S.; Pesti, Jaan A.; Yin, Jianguo. Rearrangement of Nα-Protected L-Asparagines with Iodosobenzene Diacetate. A Practical Route to β-Amino-L-alanine Derivatives. J. Org. Chem. 1997, 62 (20): 6918–6920. doi:10.1021/jo9702756.
- ^ Concepción, José I.; Francisco, Cosme G.; Hernández, Rosendo; Salazar, José A.; Suárez, Ernesto. Intramolecular hydrogen abstraction. Iodosobenzene diacetate, an efficient and convenient reagent for alkoxy radical generation. Tetrahedron Letters. 1984, 25 (18): 1953–1956. doi:10.1016/S0040-4039(01)90085-1.
- ^ Courtneidge, John L.; Lusztyk, Janusz; Pagé, Daniel. Alkoxyl radicals from alcohols. Spectroscopic detection of intermediate alkyl and acyl hypoiodites in the Suárez and Beebe reactions. Tetrahedron Letters. 1994, 35 (7): 1003–1006. doi:10.1016/S0040-4039(00)79950-3.
- ^ Dorta, R. L.; Francisco, C.G.; Freire, R.; Suárez, E. Intramolecular hydrogen abstraction. The use of organoselenium reagents for the generation of alkoxy radicals. Tetrahedron Letters. 1988, 29 (42): 5429–5432. doi:10.1016/S0040-4039(00)82887-7.
- ^ Condakes, Matthew L.; Hung, Kevin; Harwood, Stephen J.; Maimone, Thomas J. Total Syntheses of (−)-Majucin and (−)-Jiadifenoxolane A, Complex Majucin-Type Illicium Sesquiterpenes. Journal of the American Chemical Society. 2017, 139 (49): 17783–17786. doi:10.1021/jacs.7b11493.
- ^ Qing, Zhineng; Mao, Peng; Wang, Tie; Zhai, Hongbin. Asymmetric Total Syntheses of Cephalotane-Type Diterpenoids Cephanolides A–D. Journal of the American Chemical Society. 2022, 144 (23): 10640–10646. doi:10.1021/jacs.2c03978.