匹那西泮
化合物
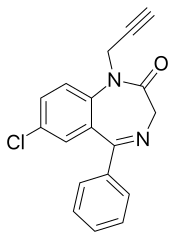
匹那西泮(英語:Pinazepam),商品名為Domar和Duna,是一種苯二氮䓬類藥物。[1]它具有抗焦慮、抗驚厥、鎮靜和骨骼肌鬆弛作用。[2]
 | |
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | 9-chloro-6-phenyl-2-prop-2-ynyl-2,5-diazabicyclo[5.4.0]undeca-5,8,10,12-tetraen-3-one |
| AHFS/Drugs.com | 國際藥品名稱 |
| 給藥途徑 | Oral |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 藥物代謝 | Hepatic |
| 排泄途徑 | Renal |
| 識別資訊 | |
| |
| CAS號 | 52463-83-9 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.052.650 |
| 化學資訊 | |
| 化學式 | C18H13ClN2O |
| 摩爾質量 | 308.8 |
| 3D模型(JSmol) | |
| |
| |
匹那西泮及其代謝物去甲西泮在子宮內轉移至發育中的胎兒,但母親血漿藥物水平通常顯著高於胎兒。[3]
匹那西泮與其他苯二氮䓬類藥物的不同之處在於它在苯二氮䓬結構的N-1位具有炔丙基。它的毒性低於地西泮,在動物研究中,它似乎具有抗焦慮和抗激動的特性,具有有限的睡眠和運動協調損害特性。[4][5]匹那西泮在口服後被吸收迅速。匹那西泮的主要活性代謝產物是去炔丙基匹那西泮(即去甲西泮)和奧沙西泮。[6]在人體中,匹那西泮作為一種純抗焦慮劑發揮作用,因為它不具有任何顯著的苯二氮䓬類藥物的其他藥理學特性。匹那西泮不會對智力、運動和睡眠造成損害,因此它比其他苯二氮䓬類藥物更適合在白天使用。[7][8][9]老年人的消除半衰期較長。[10]
參見
編輯參考資料
編輯- ^ Schütz H, Holland EM, Kazemian-Erdmann F, Schölermann K. [Screening of the new benzodiazepine derivative, pinazepan, and its major metabolites]. Arzneimittel-Forschung. September 1988, 38 (9): 1372–5. PMID 3146986.
- ^ Janbroers, J. M. Pinazepam: review of pharmacological properties and therapeutic efficacy. Clinical Therapeutics. 1984, 6 (4): 434–450 [2023-01-25]. ISSN 0149-2918. PMID 6147192. (原始內容存檔於2023-01-25).
- ^ Pacifici GM, Cuoci L, Guarneri M, Fornaro P, Arcidiacono G, Cappelli N, et al. Placental transfer of pinazepam and its metabolite N-desmethyldiazepam in women at term. European Journal of Clinical Pharmacology. 1984, 27 (3): 307–10. PMID 6150857. S2CID 1389302. doi:10.1007/BF00542165.
- ^ Universal Guide to Diazepam. fastukmeds.to. [2021-08-23].
- ^ Diazepam Injection BP - Summary of Product Characteristics (SmPC) - (emc). www.medicines.org.uk. [2021-08-23]. (原始內容存檔於2023-01-25).
- ^ Dinis-Oliveira, Ricardo Jorge. Metabolic profile of oxazepam and related benzodiazepines: clinical and forensic aspects. Drug Metabolism Reviews. Sep 14, 2017, 49 (4): 451–463 [2023-01-25]. ISSN 1097-9883. PMID 28903606. S2CID 4528255. doi:10.1080/03602532.2017.1377223. (原始內容存檔於2023-01-25).
- ^ Janbroers JM. Pinazepam: review of pharmacological properties and therapeutic efficacy. Clinical Therapeutics. 1984, 6 (4): 434–50. PMID 6147192.
- ^ Pacifici GM, Placidi GF, Fornaro P, Gomeni R. Pharmacokinetics of pinazepam in healthy volunteers. International Journal of Clinical Pharmacology Research. 1983, 3 (5): 331–7. PMID 6147314.
- ^ Pacifici GM, Placidi GF, Fornaro P, Gomeni R. Pinazepam: a precursor of N-desmethyldiazepam. European Journal of Clinical Pharmacology. 1982, 22 (3): 225–8. PMID 6809477. S2CID 1977572. doi:10.1007/BF00545219.
- ^ Pacifici GM, Cuoci L, Placidi GF, Fornaro P, Gomeni R. Elimination kinetics of desmethyldiazepam in two young and two elderly subjects. European Journal of Drug Metabolism and Pharmacokinetics. Jan 1982, 7 (1): 69–72. PMID 6802645. S2CID 21836038. doi:10.1007/bf03189546.