用户:ErwinTATP/沙盒1
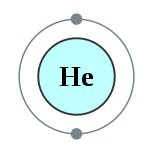
氦是一种化学元素,元素符号为He,原子序数为2. 它是一种无色、无臭、无味、无毒、惰性的单原子气体,在元素周期表中位于稀有气体的最上方。氦的熔点和沸点是所有元素中最低的,因此除某些极端情况外,氦均以气体形态存在。
氦是原子量第二低的元素,也是已知宇宙中丰度第二高的元素。宇宙中氦的质量占宇宙元素总质量的24%,相当于所有原子序数更高的元素的总质量的12倍多;它在太阳和木星中的丰度和在宇宙中的丰度相近。氦-4每个核子的平均核束缚能远高过排在氦之后的三个元素(锂、铍、硼),因此拥有很高的丰度。氦的高束缚能也可以解释为什么它既是核聚变产物也是放射性衰变的产物。宇宙中大多数的氦是氦-4,由宇宙大爆炸和恒星中的氢核聚变生成。
氦的英文名称Helium 来自希腊神话中的太阳神赫利俄斯。法国天文学家皮埃尔·让森在1868年一次日食时,在太阳光中发现了未知的黄色谱线,首次检测到这种元素。英国的约瑟夫·诺曼·洛克耶同样在这次日食中观测到了这条黄色谱线,提出这条谱线来自一种新的元素,并为该元素命名。氦的正式发现是在1895年:两位瑞典化学家皮·特奥多尔·克利夫和尼尔斯·朗勒特发现钇铀矿释放了氦气。1903年,美国部分地区的天然气田中发现了大量的氦;美国至今仍是全球最大的氦生产国。
氦在低温物理学中的使用(特别是用于超导磁体的冷却)约占氦产量的四分之一,是氦最主要的一种用途;最主要的商业应用是在MRI扫描仪中。氦在工业上同样有广泛用途:氦可以用做增压气和充换气,也可以做电弧焊或生长硅晶圆等过程中的保护气。这些工业用途总共消耗氦产量的一半。氦可充在气球和飞艇中作为上升气,这种一种用途所占比例较少但较为知名。[2]由于氦的密度和空气不同,吸入少量的氦会引发人声的频率和品质变化。在科研中,氦的两种液相(氦I和氦II)的行为对量子力学的研究(尤其是超流体方面的研究)十分重要。关于接近绝对零度时的物质性质(例如超导)的研究同样要使用氦。
氦在地球上相对稀有,仅占大气层体积的0.00052%.今天地层中大多数氦是由重元素(例如铀、钍)的天然α衰变产生的。衰变产生的α粒子就是氦-4的原子核,被天然气捕获。天然气中的氦最多可占总体积的7%,可通过低温分馏方法将其分离出来。氦是不可再生能源,如果将氦释放到大气层中,它会逃逸至太空。[3][4][5]
History 编辑
科学发现 编辑
首个证明氦存在的证据是太阳色球的发射光谱中的一条亮黄色谱线。1868年8月18日,法国天文学家皮埃尔·让森在印度的贡土尔观测日全食时,发现了这条波长为587.49 nm的谱线。[6][7]起初人们推测这条谱线来自钠。同年10月20日,英国天文学家约瑟夫·诺曼·洛克耶在太阳光谱中发现了一条黄线。由于这条谱线的波长和夫朗和斐谱线中钠产生的D1 线和 D2的波长相似,洛克耶将其命名为D3线。[8]他还提出这条谱线来自太阳上的一种尚未在地球上发现的元素。洛克耶和英国化学家爱德华·弗兰克兰以希腊语中的ἥλιος (helios,意为“太阳”)一词,将这一元素命名为Helium.[9][10][11]
1882年,意大利物理学家路易吉·帕尔米耶里在分析维苏威火山的岩浆时发现了氦的D3线,这是氦在地球上的首次发现纪录。[12]
1895年3月26日,苏格兰化学家威廉·拉姆齐爵士将钇铀矿(一种沥青铀矿,其质量的10%为 稀土元素)用酸处理,首次在地球上分离出氦。拉姆齐当时在寻找氩,他用硫酸处理矿物,分离出释放出的气体中的氮和氧。在剩下的气体中,他发现了一条和太阳光谱中的 D3谱线吻合的黄色谱线。[8][13][14][15]洛克耶和英国物理学家威廉·克鲁克斯鉴定了这一气体样品,证明了它是氦气。同一年,两位化学家皮·特奥多尔·克利夫和尼尔斯·朗勒特在瑞典乌普萨拉独立从钇铀矿中分离出氦;他们收集的氦足以测定这一元素的原子量。[7][16][17]在拉姆齐分离氦之前,美国地质化学家威廉·弗朗西斯·希尔布兰德同样注意到一份沥青铀矿样品中的一条不寻常的谱线,并从中分离出氦;但他认为这些谱线来自氮气。他致以拉姆齐的贺信是科学史上“发现”和“邻近发现”的一个有趣的例子。[18]
1907年,欧内斯特·卢瑟福与托马斯·罗伊兹让α粒子穿透玻璃壁进入真空管,向管中放电后观察管内气体的发射光谱,证明α粒子就是氦核。1908年,荷兰物理学家海克·卡末林·昂内斯将氦冷却至不到1K的低温,从而首次制得液态氦。[19]他还试着将氦固化,但是氦没有固、液、气三相平衡的三相点,因此他的尝试没有成功。1926年,昂内斯的学生威廉·亨德里克·科索姆在低温下向氦加压,制得了1 cm3的固态氦。[20]
1938年,苏联物理学家彼得·列昂尼多维奇·卡皮察发现氦-4在接近绝对零度时几乎没有粘度,从而发现了今天所说的超流体。[21]这一现象和玻色-爱因斯坦凝聚有关。1972年,美国物理学家道格拉斯·奥谢罗夫、戴维·李、以及罗伯特·科尔曼·理查森发现氦-3也有超流体现象,但所需的温度比氦-4低得多。氦-3的超流体现象被认为和氦-3费米子配对形成玻色子有关,这种配对和超导体中电子形成的库珀对类似。[22]
提取和使用 编辑
1903年,美国德克萨斯州德克斯特的一次钻探开采出了一口无法燃烧的气井。堪萨斯州的地质学家伊拉斯谟·霍沃思将取自这口井的气体样品带回堪萨斯大学分析,在化学家汉密尔顿·凯迪和大卫·麦克法兰的帮助下,他发现该气体中含有72%(体积分数,下同)氮气、15%甲烷、1%氢气和12%无法鉴定的气体。[7][23]凯迪和麦克法兰进一步分析后确认样品中含有1.84%的氦。[24][25]这说明尽管氦在地球上十分稀少,在北美大平原地下却有大量集中的储藏,可作为天然气的副产品提取出来。[26]
美国因此成为全球最大的氦生产国。在理查德·斯瑞弗的建议下,美国海军在一战期间赞助了三家实验性的小型制氦厂,以便给防空气球提供不可燃、比空气轻的提升气。此前全世界制得的氦气总共不到1立方米;而这三家工厂总共生产了5,700 m3 (200,000 立方英尺)纯度为92%的氦。[8]其中一部分气体用来填充美国海军的C-7飞艇。1921年12月1日,C-7飞艇进行了处女航,从弗吉尼亚州的汉普顿锚地到华盛顿特区的伯林菲尔德,成为世界第一艘氦气填充的飞艇。[27]
尽管一战时还没有用低温气体液化来纯化氦气的技术,氦气的生产依旧持续。 一战时期,氦气主要作为浮升器中的提升气使用;二战时这方面的需求提升了。此外,二战时对电弧焊所用的保护氦气的需求也有提高。氦质谱仪在美国制造原子弹的曼哈顿计划中起到了重要作用。[28]
1930年到1945年生产的氦气纯度约为98.3%(其余为氮气),这一纯度对飞艇而言已经足够。1945年有少量的99.9%纯氦气被制备用于电弧焊。到1949年,已经可获得商业使用量级的99.95%纯氦气了。[29]
1925年,美国政府在德克萨斯州阿马里洛建立了美国国家氦气储备库,以便为军用飞艇和商业飞艇提供氦气。[8]而当时德国的齐柏林飞艇(如兴登堡号)不得不使用易燃的氢气做为提升气,有如下原因:氦气的价格昂贵,美国垄断了氦气生产,而美国1927年通过的《氦气管控法案》禁止美国出口氦气。二战后的氦气需求有所收缩,但美国在50年代扩充了氦气储备库,以保障太空竞赛和冷战期间生产液氢/液氧火箭燃料所需的液氦冷冻剂供应。1965年美国的氦气使用量是战时最高使用量的的八倍多。[30]
《氦气管控法案1960年修正案》(公共法 86–777)通过后,美国矿业局安排五家私有工厂从天然气中收集氦。矿业局为这一“氦气转化”项目建造了一条长425-英里(684-千米)的输气管道,从堪萨斯州布什顿出发,将这些工厂和已经部分耗竭的克利夫赛德气田(为政府所有,位于阿马里洛附近)连接起来。工厂提取的氦-氮混合气储存在克利夫赛德气田中,当需要时再提取出来进行纯化。[31]
到了1995年,美国政府储备了10亿立方米的这种混合气,为此欠下了14亿美元的债务,促使美国议会在1996年决定逐步清空这一储备。[7][32]这一决定的结果是《1996年氦气私人化法》[33] (公共法 104–273) ,它指导美国内政部自2005年起出售储备气体,以清空储备库。[34]截至2012年,美国国家氦气储备库仍拥有全世界30%的氦气。[35]尽管美国参议院通过的一项法案能让储备库继续出售氦气,[36]预计到2018年,库内的储备才会耗尽。[35]其它的一些大的氦气储备位于美国堪萨斯州的雨果顿气田,以及位于该气田附近,分布于德克萨斯州与俄克拉荷马州的锅柄和堪萨斯州的天然气田。
很长一段时间内,美国生产了全世界商业用氦气的90%,其余的10%由加拿大、波兰、俄罗斯等国的工厂提供。20世纪90年代中叶,阿尔及利亚阿尔泽开办了一家新的氦生产厂,产量达1700万立方米,可满足整个欧洲的需求。而到了2000年,美国国内的氦气消费量已上涨至每年1.5万吨。[37]2004-2006年,卡塔尔的拉斯拉凡港和阿尔及利亚的斯基克达建立了两家新的氦厂。阿尔及利亚从此成为世界第二大氦生产国。[38]而这次,氦气的消费量和生产成本都上涨了。[39] In the 2002 to 2007 period helium prices doubled.[40]
Characteristics 编辑
The helium atom 编辑
Helium in quantum mechanics 编辑
In the perspective of quantum mechanics, helium is the second simplest atom to model, following the hydrogen atom. Helium is composed of two electrons in atomic orbitals surrounding a nucleus containing two protons along with some neutrons. As in Newtonian mechanics, no system consisting of more than two particles can be solved with an exact analytical mathematical approach (see 3-body problem) and helium is no exception. Thus, numerical mathematical methods are required, even to solve the system of one nucleus and two electrons. Such computational chemistry methods have been used to create a quantum mechanical picture of helium electron binding which is accurate to within < 2% of the correct value, in a few computational steps.[42] In such models it is found that each electron in helium partly screens the nucleus from the other, so that the effective nuclear charge Z which each electron sees, is about 1.69 units, not the 2 charges of a classic "bare" helium nucleus.
编辑
The nucleus of the helium-4 atom is identical with an alpha particle. High-energy electron-scattering experiments show its charge to decrease exponentially from a maximum at a central point, exactly as does the charge density of helium's own electron cloud. This symmetry reflects similar underlying physics: the pair of neutrons and the pair of protons in helium's nucleus obey the same quantum mechanical rules as do helium's pair of electrons (although the nuclear particles are subject to a different nuclear binding potential), so that all these fermions fully occupy 1s orbitals in pairs, none of them possessing orbital angular momentum, and each cancelling the other's intrinsic spin. Adding another of any of these particles would require angular momentum and would release substantially less energy (in fact, no nucleus with five nucleons is stable). This arrangement is thus energetically extremely stable for all these particles, and this stability accounts for many crucial facts regarding helium in nature.
For example, the stability and low energy of the electron cloud state in helium accounts for the element's chemical inertness, and also the lack of interaction of helium atoms with each other, producing the lowest melting and boiling points of all the elements.
In a similar way, the particular energetic stability of the helium-4 nucleus, produced by similar effects, accounts for the ease of helium-4 production in atomic reactions involving both heavy-particle emission, and fusion. Some stable helium-3 is produced in fusion reactions from hydrogen, but it is a very small fraction, compared with the highly favorable helium-4. The stability of helium-4 is the reason hydrogen is converted to helium-4 (not deuterium or helium-3 or heavier elements) in the Sun.[可疑] It is also partly responsible for the fact that the alpha particle is by far the most common type of baryonic particle to be ejected from atomic nuclei; in other words, alpha decay is far more common than cluster decay.
The unusual stability of the helium-4 nucleus is also important cosmologically: it explains the fact that in the first few minutes after the Big Bang, as the "soup" of free protons and neutrons which had initially been created in about 6:1 ratio cooled to the point that nuclear binding was possible, almost all first compound atomic nuclei to form were helium-4 nuclei. So tight was helium-4 binding that helium-4 production consumed nearly all of the free neutrons in a few minutes, before they could beta-decay, and also leaving few to form heavier atoms such as lithium, beryllium, or boron. Helium-4 nuclear binding per nucleon is stronger than in any of these elements (see nucleogenesis and binding energy) and thus no energetic drive was available, once helium had been formed, to make elements 3, 4 and 5. It was barely energetically favorable for helium to fuse into the next element with a lower energy per nucleon, carbon. However, due to lack of intermediate elements, this process requires three helium nuclei striking each other nearly simultaneously (see triple alpha process). There was thus no time for significant carbon to be formed in the few minutes after the Big Bang, before the early expanding universe cooled to the temperature and pressure point where helium fusion to carbon was no longer possible. This left the early universe with a very similar ratio of hydrogen/helium as is observed today (3 parts hydrogen to 1 part helium-4 by mass), with nearly all the neutrons in the universe trapped in helium-4.
All heavier elements (including those necessary for rocky planets like the Earth, and for carbon-based or other life) have thus been created since the Big Bang in stars which were hot enough to fuse helium itself. All elements other than hydrogen and helium today account for only 2% of the mass of atomic matter in the universe. Helium-4, by contrast, makes up about 23% of the universe's ordinary matter—nearly all the ordinary matter that is not hydrogen.
Gas and plasma phases 编辑
Helium is the second least reactive noble gas, after neon, and thus the second least reactive of all elements.[43] It is inert and monatomic in all standard conditions. Because of helium's relatively low molar (atomic) mass, its thermal conductivity, specific heat, and sound speed in the gas phase are all greater than any other gas except hydrogen. For similar reasons, and also due to the small size of helium atoms, helium's diffusion rate through solids is three times that of air and around 65% that of hydrogen.[8]
Helium is the least water soluble monatomic gas,[44] and one of the least water soluble of any gas (CF4, SF6, and C4F8 have lower mole fraction solubilities: 0.3802, 0.4394, and 0.2372 x2/10−5, respectively, versus helium's 0.70797 x2/10−5),[45] and helium's index of refraction is closer to unity than that of any other gas.[46] Helium has a negative Joule-Thomson coefficient at normal ambient temperatures, meaning it heats up when allowed to freely expand. Only below its Joule-Thomson inversion temperature (of about 32 to 50 K at 1 atmosphere) does it cool upon free expansion.[8] Once precooled below this temperature, helium can be liquefied through expansion cooling.
Most extraterrestrial helium is found in a plasma state, with properties quite different from those of atomic helium. In a plasma, helium's electrons are not bound to its nucleus, resulting in very high electrical conductivity, even when the gas is only partially ionized. The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind together with ionized hydrogen, the particles interact with the Earth's magnetosphere giving rise to Birkeland currents and the aurora.[47]
Solid, liquid, and superfluid phases 编辑
Unlike any other element, helium will remain liquid down to absolute zero at normal pressures. This is a direct effect of quantum mechanics: specifically, the zero point energy of the system is too high to allow freezing. Solid helium requires a temperature of 1–1.5 K (about −272 °C or −457 °F) and about 25 bar (2.5 MPa) of pressure.[48] It is often hard to distinguish solid from liquid helium since the refractive index of the two phases are nearly the same. The solid has a sharp melting point and has a crystalline structure, but it is highly compressible; applying pressure in a laboratory can decrease its volume by more than 30%.[49] With a bulk modulus of about 27 MPa[50] it is ~100 times more compressible than water. Solid helium has a density of 0.214 ± 0.006 g/cm3 at 1.15 K and 66 atm; the projected density at 0 K and 25 bar (2.5 MPa) is 0.187 ± 0.009 g/cm3.[51]
Helium I state 编辑
Below its boiling point of 4.22 kelvins and above the lambda point of 2.1768 kelvins, the isotope helium-4 exists in a normal colorless liquid state, called helium I.[8] Like other cryogenic liquids, helium I boils when it is heated and contracts when its temperature is lowered. Below the lambda point, however, helium does not boil, and it expands as the temperature is lowered further.
Helium I has a gas-like index of refraction of 1.026 which makes its surface so hard to see that floats of styrofoam are often used to show where the surface is.[8] This colorless liquid has a very low viscosity and a density of 0.145–0.125 g/mL (between about 0 and 4 K),[52] which is only one-fourth the value expected from classical physics.[8] Quantum mechanics is needed to explain this property and thus both types of liquid helium are called quantum fluids, meaning they display atomic properties on a macroscopic scale. This may be an effect of its boiling point being so close to absolute zero, preventing random molecular motion (thermal energy) from masking the atomic properties.[8]
Helium II state 编辑
Liquid helium below its lambda point begins to exhibit very unusual characteristics, in a state called helium II. When helium II boils, due to its high thermal conductivity it does not bubble but rather evaporates directly from its surface. Helium-3 also has a superfluid phase, but only at much lower temperatures; as a result, less is known about such properties in the isotope.[8]
Helium II is a superfluid, a quantum mechanical state (see: macroscopic quantum phenomena) of matter with strange properties . For example, when it flows through capillaries as thin as 10−7 to 10−8 m it has no measurable viscosity.[7] However, when measurements were done between two moving discs, a viscosity comparable to that of gaseous helium was observed. Current theory explains this using the two-fluid model for helium II. In this model, liquid helium below the lambda point is viewed as containing a proportion of helium atoms in a ground state, which are superfluid and flow with exactly zero viscosity, and a proportion of helium atoms in an excited state, which behave more like an ordinary fluid.[53]
In the fountain effect, a chamber is constructed which is connected to a reservoir of helium II by a sintered disc through which superfluid helium leaks easily but through which non-superfluid helium cannot pass. If the interior of the container is heated, the superfluid helium changes to non-superfluid helium. In order to maintain the equilibrium fraction of superfluid helium, superfluid helium leaks through and increases the pressure, causing liquid to fountain out of the container.[54]
The thermal conductivity of helium II is greater than that of any other known substance, a million times that of helium I and several hundred times that of copper.[8] This is because heat conduction occurs by an exceptional quantum mechanism. Most materials that conduct heat well have a valence band of free electrons which serve to transfer the heat. Helium II has no such valence band but nevertheless conducts heat well. The flow of heat is governed by equations that are similar to the wave equation used to characterize sound propagation in air. When heat is introduced, it moves at 20 meters per second at 1.8 K through helium II as waves in a phenomenon known as second sound.[8]
Helium II also exhibits a creeping effect. When a surface extends past the level of helium II, the helium II moves along the surface, against the force of gravity. Helium II will escape from a vessel that is not sealed by creeping along the sides until it reaches a warmer region where it evaporates. It moves in a 30 nm-thick film regardless of surface material. This film is called a Rollin film and is named after the man who first characterized this trait, Bernard V. Rollin.[8][55][56] As a result of this creeping behavior and helium II's ability to leak rapidly through tiny openings, it is very difficult to confine liquid helium. Unless the container is carefully constructed, the helium II will creep along the surfaces and through valves until it reaches somewhere warmer, where it will evaporate. Waves propagating across a Rollin film are governed by the same equation as gravity waves in shallow water, but rather than gravity, the restoring force is the van der Waals force.[57] These waves are known as third sound.[58]
Isotopes 编辑
There are eight known isotopes of helium, but only helium-3 and helium-4 are stable. In the Earth's atmosphere, there is one 3
He atom for every million 4
He atoms.[7] Unlike most elements, helium's isotopic abundance varies greatly by origin, due to the different formation processes. The most common isotope, helium-4, is produced on Earth by alpha decay of heavier radioactive elements; the alpha particles that emerge are fully ionized helium-4 nuclei. Helium-4 is an unusually stable nucleus because its nucleons are arranged into complete shells. It was also formed in enormous quantities during Big Bang nucleosynthesis.[59]
Helium-3 is present on Earth only in trace amounts; most of it since Earth's formation, though some falls to Earth trapped in cosmic dust.[60] Trace amounts are also produced by the beta decay of tritium.[61] Rocks from the Earth's crust have isotope ratios varying by as much as a factor of ten, and these ratios can be used to investigate the origin of rocks and the composition of the Earth's mantle.[60] 3
He is much more abundant in stars, as a product of nuclear fusion. Thus in the interstellar medium, the proportion of 3
He to 4
He is around 100 times higher than on Earth.[62] Extraplanetary material, such as lunar and asteroid regolith, have trace amounts of helium-3 from being bombarded by solar winds. The Moon's surface contains helium-3 at concentrations on the order of 0.01 ppm, much higher than the ca. 5 ppt found in the Earth's atmosphere.[63][64] A number of people, starting with Gerald Kulcinski in 1986,[65] have proposed to explore the moon, mine lunar regolith and use the helium-3 for fusion.
Liquid helium-4 can be cooled to about 1 kelvin using evaporative cooling in a 1-K pot. Similar cooling of helium-3, which has a lower boiling point, can achieve about 0.2 kelvin in a helium-3 refrigerator. Equal mixtures of liquid 3
He and 4
He below 0.8 K separate into two immiscible phases due to their dissimilarity (they follow different quantum statistics: helium-4 atoms are bosons while helium-3 atoms are fermions).[8] Dilution refrigerators use this immiscibility to achieve temperatures of a few millikelvins.
It is possible to produce exotic helium isotopes, which rapidly decay into other substances. The shortest-lived heavy helium isotope is helium-5 with a half-life of 7.6×10−22 s. Helium-6 decays by emitting a beta particle and has a half-life of 0.8 second. Helium-7 also emits a beta particle as well as a gamma ray. Helium-7 and helium-8 are created in certain nuclear reactions.[8] Helium-6 and helium-8 are known to exhibit a nuclear halo.[8]
Compounds 编辑
Helium has a valence of zero and is chemically unreactive under all normal conditions.[49] It is an electrical insulator unless ionized. As with the other noble gases, helium has metastable energy levels that allow it to remain ionized in an electrical discharge with a voltage below its ionization potential.[8] Helium can form unstable compounds, known as excimers, with tungsten, iodine, fluorine, sulfur and phosphorus when it is subjected to a glow discharge, to electron bombardment, or else is a plasma for another reason. The molecular compounds HeNe, HgHe10, and WHe2, and the molecular ions He+
2, He2+
2, HeH+
, and HeD+
have been created this way.[66] HeH+ is also stable in its ground state, but is extremely reactive—it is the strongest Brønsted acid known, and therefore can exist only in isolation, as it will protonate any molecule or counteranion it comes into contact with. This technique has also allowed the production of the neutral molecule He2, which has a large number of band systems, and HgHe, which is apparently held together only by polarization forces.[8] Theoretically, other true compounds may also be possible, such as helium fluorohydride (HHeF) which would be analogous to HArF, discovered in 2000.[67] Calculations show that two new compounds containing a helium-oxygen bond could be stable.[68] Two new molecular species, predicted using theory, CsFHeO and N(CH3)4FHeO, are derivatives of a metastable [F– HeO] anion first theorized in 2005 by a group from Taiwan. If confirmed by experiment, the only remaining element with no known stable compounds would be neon.[69]
Helium has been put inside the hollow carbon cage molecules (the fullerenes) by heating under high pressure. The endohedral fullerene molecules formed are stable up to high temperatures. When chemical derivatives of these fullerenes are formed, the helium stays inside.[70] If helium-3 is used, it can be readily observed by helium nuclear magnetic resonance spectroscopy.[71] Many fullerenes containing helium-3 have been reported. Although the helium atoms are not attached by covalent or ionic bonds, these substances have distinct properties and a definite composition, like all stoichiometric chemical compounds.
Occurrence and production 编辑
Natural abundance 编辑
Although it is rare on Earth, Helium is the second most abundant element in the known Universe (after hydrogen), constituting 23% of its baryonic mass.[7] The vast majority of helium was formed by Big Bang nucleosynthesis one to three minutes after the Big Bang. As such, measurements of its abundance contribute to cosmological models. In stars, it is formed by the nuclear fusion of hydrogen in proton-proton chain reactions and the CNO cycle, part of stellar nucleosynthesis.[59]
In the Earth's atmosphere, the concentration of helium by volume is only 5.2 parts per million.[72][73] The concentration is low and fairly constant despite the continuous production of new helium because most helium in the Earth's atmosphere escapes into space by several processes.[74][75][76] In the Earth's heterosphere, a part of the upper atmosphere, helium and other lighter gases are the most abundant elements.
Most helium on Earth is a result of radioactive decay. Helium is found in large amounts in minerals of uranium and thorium, including cleveite, pitchblende, carnotite and monazite, because they emit alpha particles (helium nuclei, He2+) to which electrons immediately combine as soon as the particle is stopped by the rock. In this way an estimated 3000 metric tons of helium are generated per year throughout the lithosphere.[77][78][79] In the Earth's crust, the concentration of helium is 8 parts per billion. In seawater, the concentration is only 4 parts per trillion. There are also small amounts in mineral springs, volcanic gas, and meteoric iron. Because helium is trapped in the subsurface under conditions that also trap natural gas, the greatest natural concentrations of helium on the planet are found in natural gas, from which most commercial helium is extracted. The concentration varies in a broad range from a few ppm up to over 7% in a small gas field in San Juan County, New Mexico.[80][81]
Modern extraction and distribution 编辑
For large-scale use, helium is extracted by fractional distillation from natural gas, which can contain up to 7% helium.[82] Since helium has a lower boiling point than any other element, low temperature and high pressure are used to liquefy nearly all the other gases (mostly nitrogen and methane). The resulting crude helium gas is purified by successive exposures to lowering temperatures, in which almost all of the remaining nitrogen and other gases are precipitated out of the gaseous mixture. Activated charcoal is used as a final purification step, usually resulting in 99.995% pure Grade-A helium.[8] The principal impurity in Grade-A helium is neon. In a final production step, most of the helium that is produced is liquefied via a cryogenic process. This is necessary for applications requiring liquid helium and also allows helium suppliers to reduce the cost of long distance transportation, as the largest liquid helium containers have more than five times the capacity of the largest gaseous helium tube trailers.[38][83]
In 2008, approximately 169 million standard cubic meters (SCM) of helium were extracted from natural gas or withdrawn from helium reserves with approximately 78% from the United States, 10% from Algeria, and most of the remainder from Russia, Poland and Qatar.[84] In the United States, most helium is extracted from natural gas of the Hugoton and nearby gas fields in Kansas, Oklahoma, and the Panhandle Field in Texas.[85][38] Much of this gas was once sent by pipeline to the National Helium Reserve, but since 2005 this reserve is presently being depleted and sold off.
Diffusion of crude natural gas through special semipermeable membranes and other barriers is another method to recover and purify helium.[86] In 1996, the U.S. had proven helium reserves, in such gas well complexes, of about 147 billion standard cubic feet (4.2 billion SCM).[87] At rates of use at that time (72 million SCM per year in the U.S.; see pie chart below) this is enough helium for about 58 years of U.S. use, and less than this (perhaps 80% of the time) at world use rates, although factors in saving and processing impact effective reserve numbers. It is estimated that the resource base for yet-unproven helium in natural gas in the U.S. is 31–53 trillion SCM, about 1000 times the proven reserves.[88]
Helium must be extracted from natural gas because it is present in air at only a fraction of that of neon, yet the demand for it is far higher. It is estimated that if all neon production were retooled to save helium, that 0.1% of the world's helium demands would be satisfied. Similarly, only 1% of the world's helium demands could be satisfied by re-tooling all air distillation plants.[89] Helium can be synthesized by bombardment of lithium or boron with high-velocity protons, but this process is a completely uneconomic method of production.[90]
Helium is commercially available in either liquid or gaseous form. As a liquid, it can be supplied in small insulated containers called dewars which hold up to 1,000 liters of helium, or in large ISO containers which have nominal capacities as large as 42 m3 (around 11,000 U.S. gallons). In gaseous form, small quantities of helium are supplied in high-pressure cylinders holding up to 8 m3 (approx. 282 standard cubic feet), while large quantities of high-pressure gas are supplied in tube trailers which have capacities of up to 4,860 m3 (approx. 172,000 standard cubic feet).
Conservation advocates 编辑
According to helium conservationists like Robert Coleman Richardson, the free market price of helium has contributed to "wasteful" usage (e.g. for helium balloons). Prices in the 2000s have been lowered by U.S. Congress' decision to sell off the country's large helium stockpile by 2015.[91] According to Richardson, the current price needs to be multiplied by 20 to eliminate the excessive wasting of helium. In their book, the Future of helium as a natural resource (Routledge, 2012), Nuttall, Clarke & Glowacki (2012) also proposed to create an International Helium Agency (IHA) to build a sustainable market for this precious commodity.[92]
Applications 编辑
While balloons are perhaps the most well-known use of helium, they are a minor part of all helium use.[32] Helium is used for many purposes that require some of its unique properties, such as its low boiling point, low density, low solubility, high thermal conductivity, or inertness. Of the 2008 world helium total production of about 32 million kg (193 million standard cubic meters) helium per year, the largest use (about 22% of the total in 2008) is in cryogenic applications, most of which involves cooling the superconducting magnets in medical MRI scanners.[93] Other major uses (totalling to about 60% of use in 1996) were pressurizing and purging systems, maintenance of controlled atmospheres, welding, and leak detection. Other uses by category were relatively minor fractions.[94]
Controlled atmospheres 编辑
Helium is used as a protective gas in growing silicon and germanium crystals, in titanium and zirconium production, and in gas chromatography,[49] because it is inert. Because of its inertness, thermally and calorically perfect nature, high speed of sound, and high value of the heat capacity ratio, it is also useful in supersonic wind tunnels[95] and impulse facilities.[96]
Gas tungsten arc welding 编辑
Helium is used as a shielding gas in arc welding processes on materials that at welding temperatures are contaminated and weakened by air or nitrogen.[7] A number of inert shielding gases are used in gas tungsten arc welding, but helium is used instead of cheaper argon especially for welding materials that have higher heat conductivity, like aluminium or copper.
Minor uses 编辑
Industrial leak detection 编辑
One industrial application for helium is leak detection. Because helium diffuses through solids three times faster than air, it is used as a tracer gas to detect leaks in high-vacuum equipment (such as cryogenic tanks) and high-pressure containers.[97] The tested object is placed in a chamber, which is then evacuated and filled with helium. The helium that escapes through the leaks is detected by a sensitive device (helium mass spectrometer), even at the leak rates as small as 10−9 mbar·L/s (10−10 Pa·m3/s). The measurement procedure is normally automatic and is called helium integral test. A simpler procedure is to fill the tested object with helium and to manually search for leaks with a hand-held device.[98]
Helium leaks through cracks should not be confused with gas permeation through a bulk material. While helium has documented permeation constants (thus a calculable permeation rate) through glasses, ceramics, and synthetic materials, inert gases such as helium will not permeate most bulk metals.[99]
Flight 编辑
Because it is lighter than air, airships and balloons are inflated with helium for lift. While hydrogen gas is approximately 7% more buoyant,[来源请求] helium has the advantage of being non-flammable (in addition to being fire retardant). Another minor use is in rocketry, where helium is used as an ullage medium to displace fuel and oxidizers in storage tanks and to condense hydrogen and oxygen to make rocket fuel. It is also used to purge fuel and oxidizer from ground support equipment prior to launch and to pre-cool liquid hydrogen in space vehicles. For example, the Saturn V booster used in the Apollo program needed about 370,000 m3 (13 million cubic feet) of helium to launch.[49]
Minor commercial and recreational uses 编辑
For its low solubility in nervous tissue, helium mixtures such as trimix, heliox and heliair are used for deep diving to reduce the effects of narcosis.[100][101] At depths below 150米(490英尺) small amounts of hydrogen[来源请求] are added to a helium-oxygen mixture to counter the effects of high-pressure nervous syndrome.[102] At these depths the low density of helium is found to considerably reduce the effort of breathing.[103]
Helium-neon lasers, a type of low-powered gas laser producing a red beam, had various practical applications which included barcode readers and laser pointers, before they were almost universally replaced by cheaper diode lasers.[7]
For its inertness and high thermal conductivity, neutron transparency, and because it does not form radioactive isotopes under reactor conditions, helium is used as a heat-transfer medium in some gas-cooled nuclear reactors.[97]
Helium, mixed with a heavier gas such as xenon, is useful for thermoacoustic refrigeration due to the resulting high heat capacity ratio and low Prandtl number.[104] The inertness of helium has environmental advantages over conventional refrigeration systems which contribute to ozone depletion or global warming.[105]
Scientific uses 编辑
The use of helium reduces the distorting effects of temperature variations in the space between lenses in some telescopes, due to its extremely low index of refraction.[8] This method is especially used in solar telescopes where a vacuum tight telescope tube would be too heavy.[106][107]
Helium is a commonly used carrier gas for gas chromatography.
The age of rocks and minerals that contain uranium and thorium can be estimated by measuring the level of helium with a process known as helium dating.[7][8]
Helium at low temperatures is used in cryogenics, and in certain cryogenics applications. As examples of applications, liquid helium is used to cool certain metals to the extremely low temperatures required for superconductivity, such as in superconducting magnets for magnetic resonance imaging. The Large Hadron Collider at CERN uses 96 metric tons of liquid helium to maintain the temperature at 1.9 kelvin.[108]
Inhalation and safety 编辑
Neutral helium at standard conditions is non-toxic, plays no biological role and is found in trace amounts in human blood.
The speed of sound in helium is nearly three times the speed of sound in air. Because the fundamental frequency of a gas-filled cavity is proportional to the speed of sound in the gas, when helium is inhaled there is a corresponding increase in the resonant frequencies of the vocal tract.[7][109] The fundamental frequency (sometimes called pitch) does not change, since this is produced by direct vibration of the vocal folds, which is unchanged.[110] However, the higher resonant frequencies cause a change in timbre, resulting in a reedy, duck-like vocal quality. The opposite effect, lowering resonant frequencies, can be obtained by inhaling a dense gas such as sulfur hexafluoride or xenon.
Inhaling helium can be dangerous if done to excess, since helium is a simple asphyxiant and so displaces oxygen needed for normal respiration.[7][111] Breathing pure helium continuously causes death by asphyxiation within minutes. This fact is utilized in the design of suicide bags.
Inhaling helium directly from pressurized cylinders is extremely dangerous, as the high flow rate can result in barotrauma, fatally rupturing lung tissue.[111][112] However, death caused by helium is rare, with only two fatalities reported between 2000 and 2004 in the United States.[112] However, there were two cases in 2010, one in the USA[113] in January and another in Northern Ireland in November.[114] An Oregon girl died in 2012 from barotrauma,[115] and an another girl from hypoxia later in the year.[116]
The safety issues for cryogenic helium are similar to those of liquid nitrogen; its extremely low temperatures can result in cold burns and the liquid-to-gas expansion ratio can cause explosions if no pressure-relief devices are installed. Containers of helium gas at 5 to 10 K should be handled as if they contain liquid helium due to the rapid and significant thermal expansion that occurs when helium gas at less than 10 K is warmed to room temperature.[49]
At high pressures (more than about 20 atm or two MPa), a mixture of helium and oxygen (heliox) can lead to high-pressure nervous syndrome, a sort of reverse-anesthetic effect; adding a small amount of nitrogen to the mixture can alleviate the problem.[117][118]
See also 编辑
References 编辑
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ^ Helium: Up, Up and Away? Melinda Rose, Photonics Spectra, Oct. 2008. Accessed Feb 27, 2010.
- ^ Connor, Steve. Why the world is running out of helium – Science – News. The Independent. 2010-08-23 [2013-09-16].
- ^ Posted by Ethan on December 12, 2012. Why the World Will Run Out of Helium – Starts With A Bang. Scienceblogs.com. 2012-12-12 [2013-09-16].
- ^ Witchalls, Clint (18 August 2010) Nobel prizewinner: We are running out of helium. New Scientist.
- ^ Kochhar, R. K. French astronomers in India during the 17th – 19th centuries. Journal of the British Astronomical Association. 1991, 101 (2): 95–100. Bibcode:1991JBAA..101...95K.
- ^ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 Emsley, John. Nature's Building Blocks. Oxford: Oxford University Press. 2001: 175–179. ISBN 0-19-850341-5.
- ^ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 8.14 8.15 8.16 8.17 8.18 8.19 8.20 8.21 8.22 Clifford A. Hampel. The Encyclopedia of the Chemical Elements. New York: Van Nostrand Reinhold. 1968: 256–268. ISBN 0-442-15598-0.
- ^ Sir Norman Lockyer – discovery of the element that he named helium" Balloon Professional Magazine, 7 August 2009.
- ^ Helium. Oxford English Dictionary. 2008 [2008-07-20].
- ^ Thomson, William. Inaugural Address of Sir William Thompson. Nature. Aug. 3, 1871, 4 (92): 261–278 [268]. Bibcode:1871Natur...4..261.. doi:10.1038/004261a0.
Frankland and Lockyer find the yellow prominences to give a very decided bright line not far from D, but hitherto not identified with any terrestrial flame. It seems to indicate a new substance, which they propose to call Helium
- ^ Stewart, Alfred Walter. Recent Advances in Physical and Inorganic Chemistry. BiblioBazaar, LLC. 2008: 201. ISBN 0-554-80513-8.
- ^ Ramsay, William. On a Gas Showing the Spectrum of Helium, the Reputed Cause of D3 , One of the Lines in the Coronal Spectrum. Preliminary Note. Proceedings of the Royal Society of London. 1895, 58 (347–352): 65–67. doi:10.1098/rspl.1895.0006.
- ^ Ramsay, William. Helium, a Gaseous Constituent of Certain Minerals. Part I. Proceedings of the Royal Society of London. 1895, 58 (347–352): 80–89. doi:10.1098/rspl.1895.0010.
- ^ Ramsay, William. Helium, a Gaseous Constituent of Certain Minerals. Part II--. Proceedings of the Royal Society of London. 1895, 59 (1): 325–330. doi:10.1098/rspl.1895.0097.
- ^ (德文) Langlet, N. A. Das Atomgewicht des Heliums. Zeitschrift für anorganische Chemie. 1895, 10 (1): 289–292. doi:10.1002/zaac.18950100130 (German).
- ^ Weaver, E.R. Bibliography of Helium Literature. Industrial & Engineering Chemistry. 1919.
- ^ Munday, Pat. John A. Garraty and Mark C. Carnes , 编. Biographical entry for W.F. Hillebrand (1853–1925), geochemist and U.S. Bureau of Standards administrator in American National Biography 10–11. Oxford University Press. 1999: 808–9; 227–8.
- ^ van Delft, Dirk. Little cup of Helium, big Science (PDF). Physics Today. 2008: 36–42 [2008-07-20]. (原始内容 (PDF)存档于June 25, 2008).
- ^ Coldest Cold. Time Inc. 1929-06-10 [2008-07-27].
- ^ Kapitza, P. Viscosity of Liquid Helium below the λ-Point. Nature. 1938, 141 (3558): 74. Bibcode:1938Natur.141...74K. doi:10.1038/141074a0.
- ^ Osheroff, D. D.; Richardson, R. C.; Lee, D. M. Evidence for a New Phase of Solid He3. Phys. Rev. Lett. 1972, 28 (14): 885–888. Bibcode:1972PhRvL..28..885O. doi:10.1103/PhysRevLett.28.885.
- ^ McFarland, D. F. Composition of Gas from a Well at Dexter, Kan. Transactions of the Kansas Academy of Science. 1903, 19: 60–62. JSTOR 3624173. doi:10.2307/3624173.
- ^ The Discovery of Helium in Natural Gas. American Chemical Society. 2004 [2008-07-20].
- ^ Cady, H.P.; McFarland, D. F. Helium in Natural Gas. Science. 1906, 24 (611): 344. Bibcode:1906Sci....24..344D. PMID 17772798. doi:10.1126/science.24.611.344.
- ^ Cady, H.P.; McFarland, D. F. Helium in Kansas Natural Gas. Transactions of the Kansas Academy of Science. 1906, 20: 80–81. JSTOR 3624645. doi:10.2307/3624645.
- ^ Emme, Eugene M. comp. (编). Aeronautics and Astronautics Chronology, 1920–1924. Aeronautics and Astronautics: An American Chronology of Science and Technology in the Exploration of Space, 1915–1960. Washington, D.C.: NASA. 1961: 11–19 [2008-07-20].
- ^ Hilleret, N. Leak Detection. S. Turner (编). CERN Accelerator School, vacuum technology: proceedings: Scanticon Conference Centre, Snekersten, Denmark, 28 May – 3 June 1999 (PDF). Geneva, Switzerland: CERN. 1999: 203–212.
At the origin of the helium leak detection method was the Manhattan Project and the unprecedented leak-tightness requirements needed by the uranium enrichment plants. The required sensitivity needed for the leak checking led to the choice of a mass spectrometer designed by Dr. A.O.C. Nier tuned on the helium mass.
- ^ Mullins, P.V.; Goodling, R. M. Helium. Bureau of Mines / Minerals yearbook 1949. 1951: 599–602 [2008-07-20].
- ^ Williamson, John G. Energy for Kansas. Transactions of the Kansas Academy of Science (Kansas Academy of Science). 1968, 71 (4): 432–438. JSTOR 3627447. doi:10.2307/3627447.
- ^ Conservation Helium Sale (PDF). Federal Register. 2005-10-06, 70 (193): 58464 [2008-07-20].
- ^ 32.0 32.1 Stwertka, Albert (1998). Guide to the Elements: Revised Edition. New York; Oxford University Press, p. 24. ISBN 0-19-512708-0
- ^ Helium Privatization Act of 1996 美国联邦公法第104–273号
- ^ Executive Summary. nap.edu. [2008-07-20].
- ^ 35.0 35.1 @tdnewcomb. There's a Helium Shortage On — and It's Affecting More than Just Balloons Time August 21, 2012. Newsfeed.time.com. 2012-08-21 [2013-09-16].
- ^ 美参议院几乎全票通过法案 避免氦供应短缺. 中国科学报. 2013-9-24 [2013-10-24].
- ^ Helium End User Statistic (PDF). U.S. Geological Survey. [2008-07-20].
- ^ 38.0 38.1 38.2 Smith, E.M.; Goodwin, T.W.; Schillinger, J. Challenges to the Worldwide Supply of Helium in the Next Decade. Advances in Cryogenic Engineering. 49. 2003, A (710): 119–138. doi:10.1063/1.1774674.
- ^ Kaplan, Karen H. Helium shortage hampers research and industry. Physics Today (American Institute of Physics). June 2007, 60 (6): 31–32. Bibcode:2007PhT....60f..31K. doi:10.1063/1.2754594.
- ^ Basu, Sourish. Yam, Philip , 编. Updates: Into Thin Air. Scientific American 297 (4) (Scientific American, Inc.). October 2007: 18 [2008-08-04].
- ^ 邢国海. 天然气提取氦气技术现状与发展. 天然气工业. 2008, 28 (8): 114–116.
- ^ Watkins, Thayer. The Old Quantum Physics of Niels Bohr and the Spectrum of Helium: A Modified Version of the Bohr Model. San Jose State University.
- ^ Lewars, Errol G. Modelling Marvels. Springer. 2008: 70–71. ISBN 1-4020-6972-3.
- ^ Weiss, Ray F. Solubility of helium and neon in water and seawater. J. Chem. Eng. Data. 1971, 16 (2): 235–241. doi:10.1021/je60049a019.
- ^ Scharlin, P.; Battino, R. Silla, E.; Tuñón, I.; Pascual-Ahuir, J. L. Solubility of gases in water: Correlation between solubility and the number of water molecules in the first solvation shell. Pure & Appl. Chem. 1998, 70 (10): 1895–1904. doi:10.1351/pac199870101895.
- ^ Stone, Jack A.; Stejskal, Alois. Using helium as a standard of refractive index: correcting errors in a gas refractometer. Metrologia. 2004, 41 (3): 189–197. Bibcode:2004Metro..41..189S. doi:10.1088/0026-1394/41/3/012.
- ^ Buhler, F.; Axford, W. I.; Chivers, H. J. A.; Martin, K. Helium isotopes in an aurora. J. Geophys. Res. 1976, 81 (1): 111–115. Bibcode:1976JGR....81..111B. doi:10.1029/JA081i001p00111.
- ^ Solid Helium. Department of Physics University of Alberta. 2005-10-05 [2008-07-20]. (原始内容存档于May 31, 2008).
- ^ 49.0 49.1 49.2 49.3 49.4 Lide, D. R. (编), CRC Handbook of Chemistry and Physics 86th, Boca Raton (FL): CRC Press, 2005, ISBN 0-8493-0486-5
- ^ Grilly, E. R. Pressure-volume-temperature relations in liquid and solid 4He. Journal of Low Temperature Physics. 1973, 11 (1–2): 33–52. Bibcode:1973JLTP...11...33G. doi:10.1007/BF00655035.
- ^ Henshaw, D. B. Structure of Solid Helium by Neutron Diffraction. Physical Review Letters. 1958, 109 (2): 328–330. Bibcode:1958PhRv..109..328H. doi:10.1103/PhysRev.109.328.
- ^ Lide, D. R. (编), CRC Handbook of Chemistry and Physics 86th, Boca Raton (FL): CRC Press: 6-120, 2005, ISBN 0-8493-0486-5
- ^ Hohenberg, P. C.; Martin, P. C. Microscopic Theory of Superfluid Helium. Annals of Physics. 2000, 281 (1–2): 636–705 12091211. Bibcode:2000AnPhy.281..636H. doi:10.1006/aphy.2000.6019.
- ^ Warner, Brent. Introduction to Liquid Helium. NASA. [2007-01-05]. (原始内容存档于2005-09-01).
- ^ Fairbank, H. A.; Lane, C. T. Rollin Film Rates in Liquid Helium. Physical Review. 1949, 76 (8): 1209–1211. Bibcode:1949PhRv...76.1209F. doi:10.1103/PhysRev.76.1209.
- ^ Rollin, B. V.; Simon, F. On the "film" phenomenon of liquid helium II. Physica. 1939, 6 (2): 219–230. Bibcode:1939Phy.....6..219R. doi:10.1016/S0031-8914(39)80013-1.
- ^ Ellis, Fred M. Third sound. Wesleyan Quantum Fluids Laboratory. 2005 [2008-07-23].
- ^ Bergman, D. Hydrodynamics and Third Sound in Thin He II Films. Physical Review. 1949, 188 (1): 370–384. Bibcode:1969PhRv..188..370B. doi:10.1103/PhysRev.188.370.
- ^ 59.0 59.1 Weiss, Achim. Elements of the past: Big Bang Nucleosynthesis and observation. Max Planck Institute for Gravitational Physics. [2008-06-23].; Coc, A.; et al. Updated Big Bang Nucleosynthesis confronted to WMAP observations and to the Abundance of Light Elements. Astrophysical Journal. 2004, 600 (2): 544. Bibcode:2004ApJ...600..544C. arXiv:astro-ph/0309480 . doi:10.1086/380121. Authors list列表中的
|first2=缺少|last2=(帮助); Authors list列表中的|first3=缺少|last3=(帮助); Authors list列表中的|first4=缺少|last4=(帮助); Authors list列表中的|first5=缺少|last5=(帮助) - ^ 60.0 60.1 Anderson, Don L.; Foulger, G. R.; Meibom, A. Helium Fundamentals. MantlePlumes.org. 2006-09-02 [2008-07-20].
- ^ Novick, Aaron. Half-Life of Tritium. Physical Review. 1947, 72 (10): 972–972. Bibcode:1947PhRv...72..972N. doi:10.1103/PhysRev.72.972.2.
- ^ Zastenker G. N. Isotopic Composition and Abundance of Interstellar Neutral Helium Based on Direct Measurements. Astrophysics. 2002, 45 (2): 131–142. Bibcode:2002Ap.....45..131Z. doi:10.1023/A:1016057812964. Authors list列表中的
|first2=缺少|last2=(帮助); Authors list列表中的|first3=缺少|last3=(帮助); Authors list列表中的|first4=缺少|last4=(帮助); Authors list列表中的|first5=缺少|last5=(帮助); Authors list列表中的|first6=缺少|last6=(帮助); Authors list列表中的|first7=缺少|last7=(帮助); Authors list列表中的|first8=缺少|last8=(帮助); Authors list列表中的|first9=缺少|last9=(帮助) - ^ Lunar Mining of Helium-3. Fusion Technology Institute of the University of Wisconsin-Madison. 2007-10-19 [2008-07-09].
- ^ Slyuta, E. N.; Abdrakhimov, A. M.; Galimov, E. M. The estimation of helium-3 probable reserves in lunar regolith (PDF). Lunar and Planetary Science XXXVIII. 2007 [2008-07-20].
- ^ Hedman, Eric R. A fascinating hour with Gerald Kulcinski. The Space Review. 2006-01-16 [2008-07-20].
- ^ Hiby, Julius W. Massenspektrographische Untersuchungen an Wasserstoff- und Heliumkanalstrahlen (H+
3, H−
2, HeH+
, HeD+
, He−
). Annalen der Physik. 1939, 426 (5): 473–487. Bibcode:1939AnP...426..473H. doi:10.1002/andp.19394260506. - ^ Wong, Ming Wah. Prediction of a Metastable Helium Compound: HHeF. Journal of the American Chemical Society. 2000, 122 (26): 6289–6290. doi:10.1021/ja9938175.
- ^ Grochala, W. On Chemical Bonding Between Helium and Oxygen. Polish Journal of Chemistry. 2009, 83: 87–122.
- ^ Collapse of helium's chemical nobility predicted by Polish chemist (PDF). [2009-05-15].
- ^ Saunders, Martin Hugo; Jiménez-Vázquez, A.; Cross, R. James; Poreda; Robert J. Stable Compounds of Helium and Neon: He@C60 and Ne@C60. Science. 1993, 259 (5100): 1428–1430. Bibcode:1993Sci...259.1428S. PMID 17801275. doi:10.1126/science.259.5100.1428.
- ^ Saunders, M.; et al. Probing the interior of fullerenes by 3He NMR spectroscopy of endohedral 3He@C60 and 3He@C70. Nature. 1994, 367 (6460): 256–258. Bibcode:1994Natur.367..256S. doi:10.1038/367256a0. Authors list列表中的
|first2=缺少|last2=(帮助); Authors list列表中的|first3=缺少|last3=(帮助); Authors list列表中的|first4=缺少|last4=(帮助); Authors list列表中的|first5=缺少|last5=(帮助); Authors list列表中的|first6=缺少|last6=(帮助) - ^ Oliver, B. M.; Bradley, James G. Helium concentration in the Earth's lower atmosphere. Geochimica et Cosmochimica Acta. 1984, 48 (9): 1759–1767. Bibcode:1984GeCoA..48.1759O. doi:10.1016/0016-7037(84)90030-9.
- ^ The Atmosphere: Introduction. JetStream – Online School for Weather. National Weather Service. 2007-08-29 [2008-07-12]. (原始内容存档于January 13, 2008).
- ^ Lie-Svendsen, Ø.; Rees, M. H. Helium escape from the terrestrial atmosphere: The ion outflow mechanism. Journal of Geophysical Research. 1996, 101 (A2): 2435–2444. Bibcode:1996JGR...101.2435L. doi:10.1029/95JA02208.
- ^ Strobel, Nick. Nick Strobel's Astronomy Notes. 2007 [2007-09-25].
|chapter=被忽略 (帮助) - ^ G. Brent Dalrymple. How Good Are Those Young-Earth Arguments?.
- ^ Cook, Melvine A. Where is the Earth's Radiogenic Helium?. Nature. 1957, 179 (4552): 213. Bibcode:1957Natur.179..213C. doi:10.1038/179213a0.
- ^ Aldrich, L. T.; Nier, Alfred O. The Occurrence of He3 in Natural Sources of Helium. Phys. Rev. 1948, 74 (11): 1590–1594. Bibcode:1948PhRv...74.1590A. doi:10.1103/PhysRev.74.1590.
- ^ Morrison, P.; Pine, J. Radiogenic Origin of the Helium Isotopes in Rock. Annals of the New York Academy of Sciences. 1955, 62 (3): 71–92. Bibcode:1955NYASA..62...71M. doi:10.1111/j.1749-6632.1955.tb35366.x.
- ^ Zartman, R. E.; Wasserburg, G. J.; Reynolds, J. H. Helium Argon and Carbon in Natural Gases. Journal of Geophysical Research. 1961, 66 (1): 277–306. Bibcode:1961JGR....66..277Z. doi:10.1029/JZ066i001p00277.
- ^ Broadhead, Ronald F. Helium in New Mexico – geology distribution resource demand and exploration possibilities (PDF). New Mexico Geology. 2005, 27 (4): 93–101 [2008-07-21].
- ^ Winter, Mark. Helium: the essentials. University of Sheffield. 2008 [2008-07-14].
- ^ Cai, Z.; et al. Modelling Helium Markets (PDF). University of Cambridge. 2007 [2008-07-14]. (原始内容 (PDF)存档于2009-03-26).
- ^ Helium (PDF). Mineral Commodity Summaries. U.S. Geological Survey: 74–75. 2009 [2009-12-19].
- ^ Pierce, A.P., Gott, G.B., and Mytton, J.W., Uranium and Helium in the Panhandle Gas Field Texas, and Adjacent Areas,Geological Survey Professional Paper 454-G, Washington:US Government Printing Office, 1964
- ^ Belyakov, V.P.; Durgar'yan, S. G.; Mirzoyan, B. A. Membrane technology—A new trend in industrial gas separation. Chemical and Petroleum Engineering. 1981, 17 (1): 19–21. doi:10.1007/BF01245721.
- ^ Committee on the Impact of Selling, see table for total proven US reserves
- ^ Committee on the Impact of Selling, See table 4.2 for the reserve estimate and page 47 for the unproven reserve estimate.
- ^ Committee on the Impact of Selling, see page 40 for the estimate of total theoretical helium production by neon and liquid air plants
- ^ Dee, P. I.; Walton E. T. S. A Photographic Investigation of the Transmutation of Lithium and Boron by Protons and of Lithium by Ions of the Heavy Isotope of Hydrogen. Proceedings of the Royal Society of London. 1933, 141 (845): 733–742. Bibcode:1933RSPSA.141..733D. doi:10.1098/rspa.1933.0151.
- ^ Richard Coleman campaigning against US Congress' decision to sell all helium supplies by 2015. Independent.co.uk. 2010-08-23 [2010-11-27].
- ^ Nuttall, William J.; Clarke Richard H. & Glowacki Bartek A. Resources: Stop squandering helium. Nature. 2012, 485 (7400): 573–575. Bibcode:2012Natur.485..573N. PMID 22660302. doi:10.1038/485573a.
- ^ Helium sell-off risks future supply, Michael Banks, Physics World, 27 January 2010. accessed February 27, 2010.
- ^ Information source is given in pie chart graph at right
- ^ Beckwith, I.E.; Miller, C. G. Aerothermodynamics and Transition in High-Speed Wind Tunnels at Nasa Langley. Annual Review of Fluid Mechanics. 1990, 22 (1): 419–439. Bibcode:1990AnRFM..22..419B. doi:10.1146/annurev.fl.22.010190.002223.
- ^ Morris, C.I. Shock Induced Combustion in High Speed Wedge Flows (PDF). Stanford University Thesis. 2001.
- ^ 97.0 97.1 Considine, Glenn D. (编). Helium. Van Nostrand's Encyclopedia of Chemistry. Wiley-Interscience: 764–765. 2005. ISBN 0-471-61525-0.
- ^ Hablanian, M. H. High-vacuum technology: a practical guide. CRC Press. 1997: 493. ISBN 0-8247-9834-1.
- ^ Ekin, Jack W. Experimental Techniques for Low-Temperature measurements. Oxford University Press. 2006. ISBN 0-19-857054-6.
- ^ Fowler, B; Ackles KN, Porlier G. Effects of inert gas narcosis on behavior—a critical review. Undersea Biomedical Research Journal. 1985, 12 (4): 369–402 [2008-06-27]. PMID 4082343.
- ^ Thomas, J. R. Reversal of nitrogen narcosis in rats by helium pressure. Undersea Biomed Res. 1976, 3 (3): 249–59 [2008-08-06]. PMID 969027.
- ^ Rostain, J. C.; Gardette-Chauffour, M. C.; Lemaire, C.; Naquet, R. Effects of a H2-He-O2 mixture on the HPNS up to 450 msw. Undersea Biomed. Res. 1988, 15 (4): 257–70 [2008-06-24]. OCLC 2068005. PMID 3212843.
- ^ Butcher, Scott J.; Jones, Richard L.; Mayne, Jonathan R.; Hartley, Timothy C.; Petersen, Stewart R. Impaired exercise ventilatory mechanics with the self-contained breathing apparatus are improved with heliox. European Journal of Applied Physiology (Netherlands: Springer). 2007, 101 (6): 659–69. PMID 17701048. doi:10.1007/s00421-007-0541-5.
- ^ Belcher, James R. Working gases in thermoacoustic engines. The Journal of the Acoustical Society of America. 1999, 105 (5): 2677–2684. Bibcode:1999ASAJ..105.2677B. PMID 10335618. doi:10.1121/1.426884. Authors list列表中的
|first2=缺少|last2=(帮助); Authors list列表中的|first3=缺少|last3=(帮助); Authors list列表中的|first4=缺少|last4=(帮助); Authors list列表中的|first5=缺少|last5=(帮助) - ^ Makhijani, Arjun; Gurney, Kevin. Mending the Ozone Hole: Science, Technology, and Policy. MIT Press. 1995. ISBN 0-262-13308-3.
- ^ Jakobsson, H. Simulations of the dynamics of the Large Earth-based Solar Telescope. Astronomical & Astrophysical Transactions. 1997, 13 (1): 35–46. Bibcode:1997A&AT...13...35J. doi:10.1080/10556799708208113.
- ^ Engvold, O.; Dunn, R.B.; Smartt, R. N.; Livingston, W. C. Tests of vacuum VS helium in a solar telescope. Applied Optics. 1983, 22 (1): 10–12. Bibcode:1983ApOpt..22...10E. PMID 20401118. doi:10.1364/AO.22.000010.
- ^ LHC: Facts and Figures (PDF). CERN. [2008-04-30]. (原始内容 (PDF)存档于2011-07-06).
- ^ Ackerman MJ, Maitland G. Calculation of the relative speed of sound in a gas mixture. Undersea Biomed Res. 1975, 2 (4): 305–10 [2008-08-09]. PMID 1226588.
- ^ Why does helium make your voice squeaky?. 2000-07-14 [2013-06-08].
- ^ 111.0 111.1 (德文) Grassberger, Martin; Krauskopf, Astrid. Suicidal asphyxiation with helium: Report of three cases Suizid mit Helium Gas: Bericht über drei Fälle. Wiener Klinische Wochenschrift. 2007, 119 (9–10): 323–325. PMID 17571238. doi:10.1007/s00508-007-0785-4 (German & English).
- ^ 112.0 112.1 Engber, Daniel. Stay Out of That Balloon!. Slate.com. 2006-06-13 [2008-07-14].
- ^ Teen Dies After Inhaling Helium. KTLA News (RIVERSIDE: ktla.com). January 6, 2010 [2010-11-19].
- ^ Tributes to 'helium death' teenager from Newtownabbey. BBC Online. 19 November 2010 [2010-11-19].
- ^ Parents of Eagle Point girl who died from inhaling helium hope to save others from same fate. 2012-02-24 [2013-06-08].
- ^ The Oxford Leader Newspaper, Sherman Publications, Inc., December 3, 2012.
- ^ Rostain J.C., Lemaire C., Gardette-Chauffour M.C., Doucet J., Naquet R. Estimation of human susceptibility to the high-pressure nervous syndrome. J Appl Physiol. 1983, 54 (4): 1063–70 [2008-08-09]. PMID 6853282.
- ^ Hunger Jr, W. L.; Bennett., P. B. The causes, mechanisms and prevention of the high-pressure nervous syndrome. Undersea Biomed. Res. 1974, 1 (1): 1–28 [2008-08-09]. OCLC 2068005. PMID 4619860.
Bibliography 编辑
- Bureau of Mines. Minerals yearbook mineral fuels Year 1965, Volume II (1967). U. S. Government Printing Office. 1967.
- Committee on the Impact of Selling the Federal Helium Reserve, Commission on Physical Sciences, Mathematics, and Applications, Commission on Engineering and Technical Systems, National Research Council. The Impact of Selling the Federal Helium Reserve. The National Academies Press. 2000 [2010-04-02]. ISBN 0-309-07038-4.
- Emsley, John. The Elements 3rd. New York: Oxford University Press. 1998. ISBN 978-0-19-855818-7.
- Mineral Information for Helium (PDF). United States Geological Survey (usgs.gov). [2007-01-05].
- Vercheval, J. The thermosphere: a part of the heterosphere. Belgian Institute for Space Aeronomy. 2003 [2008-07-12]. (原始内容存档于2005-01-01).
External links 编辑
- General
- U.S. Government's Bureau of Land Management: Sources, Refinement, and Shortage. With some history of helium.
- U.S. Geological Survey publications on helium beginning 1996: Helium
- Where is all the helium? Aga website
- It's Elemental – Helium
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Helium
- International Chemical Safety Cards – Helium; includes health and safety information regarding accidental exposures to helium
- More detail
- Helium at The Periodic Table of Videos (University of Nottingham)
- Helium at the Helsinki University of Technology; includes pressure-temperature phase diagrams for helium-3 and helium-4
- Lancaster University, Ultra Low Temperature Physics – includes a summary of some low temperature techniques
- Miscellaneous
- Physics in Speech with audio samples that demonstrate the unchanged voice pitch
- Article about helium and other noble gases
- Helium shortage
- Kramer, David. Senate bill would preserve US helium reserve: Measure would give scientists first dibs on helium should a shortage develop. Physics Today web site. May 22, 2012.
Template:E number infobox 930-949 Template:Compact periodic table Template:Chemical elements named after places