阿坎酸
阿坎酸(英语:Acamprosate)以Campral及Alglutol(戒酒妥)等品牌销售,是一种与治疗酒精使用疾患(酗酒)咨询合并使用的药物。[1][2]
 | |
 | |
| 临床资料 | |
|---|---|
| 读音 | /əˈkæmproʊseɪt/ |
| 商品名 | Campral、Alglutol,及其他 |
| 其他名称 | N-乙酰基高牛磺酸(N-Acetyl homotaurine), 阿坎酸钙 (JAN JP), 阿坎酸钙 (USAN US) |
| 怀孕分级 |
|
| 给药途径 | 口服给药[1] |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 生物利用度 | 11%[1] |
| 血浆蛋白结合率 | 可忽略不计[1] |
| 药物代谢 | 无[1] |
| 生物半衰期 | 20 - 33小时[1] |
| 排泄途径 | 肾脏[1] |
| 识别信息 | |
| |
| CAS号 | 77337-76-9 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.495 |
| 化学信息 | |
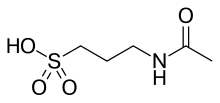
| 化学式 | C5H11NO4S |
| 摩尔质量 | 181.21 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
阿坎酸被认为可稳定大脑的神经传递,这些讯号传递会因酒精戒断而受干扰。[3]对大多数患者而言,单独使用阿坎酸并非有效的治疗方法。[4]研究发现将阿坎酸与社会心理支持结合时效果会最佳,因为该药物有助于减少饮酒数量,也有达到完全戒酒的效果。[2][5][6]
服用此药物的严重副作用有过敏反应、心律失常以及低血压或是高血压,而较不严重的副作用有头痛、失眠和勃起功能障碍。[7]最常见的副作用是腹泻。[8]目前尚不清楚个体于怀孕期间使用是否对胎儿有安全的问题。[9][10]
此药物被列入世界卫生组织基本药物标准清单之中。[11]
医疗用途
编辑有酒精使用障碍的患者在接受咨询,并同时服用阿坎酸时会非常有用。[2]在三到十二个月的治疗期间,完全不喝酒的人数和患者不喝酒的天数会增加。[2]它在维持戒酒方面似乎与纳曲酮一样有效,[12]然而纳曲酮在减少酒精渴望和酗酒方面效果稍好,[13]同时在欧洲以外,而治疗服务不太健全的地区,使用阿坎酸的效果往往会较差。[14]
用药禁忌
编辑阿坎酸主要经由肾脏排除。对于肾功能中度受损的患者(肌酸酐清除率在30毫升/分钟和50毫升/分钟之间),建议将使用剂量降低。[1][15]对药物中阿坎酸钙或其任何成分有强烈过敏反应的人也应禁止使用。[15]
不良影响
编辑此药物在美国销售的标签上会列有服用会与自杀行为、重性忧郁疾患和肾衰竭增加有关联的警告。[1]
在临床试验中,对停止服用者导致的不良反应有腹泻、恶心、忧郁和焦虑。[1]
潜在的副作用包括头痛、腹痛、背痛、肌肉痛、关节痛、胸痛、感染、类流感症状、发冷、心悸、高血压、昏厥、呕吐、胃部不适、便秘、食欲增加、体重增加、水肿、嗜睡、性欲减退、勃起功能障碍、健忘、思考障碍、视力异常、味觉异常、颤抖、流鼻涕、咳嗽、呼吸困难、喉咙痛、支气管炎和皮疹。[1]
药理学
编辑药效学
编辑阿坎酸的药效学很复杂且尚未被完全了解,[16][17][18]然而它被认为可作为NMDA受体拮抗剂和[[GABAA受体]]的正变构调节剂。[17][18]
此药物与大多数其他药物不同,其对这些受体产生的活性是间接性的。[19]抑制GABAB系统被认为会间接导致GABAA受体增强。[19]对NMDA受体的影响具有剂量依赖性,药物似乎在低浓度下可增强受体活性,而在较高浓度时会有抑制作用,可抵消患者在发生酒精戒断情况下NMDA受体过度激活状态。[20]
乙醇和苯二氮䓬类药物透过与GABAA受体结合,将具有抑制神经传导作用的GABA功能增强,而对中枢神经系统发挥作用(即此药物充当这些受体的正变构调节剂)。[17][4]对有酒精使用疾患的患者,发生耐受性的主要机制之一是GABAA受体下调(即这些受体对GABA变得不太敏感)。[4]而当个体停止饮酒后,正常的GABA生产就会导致戒断症状出现,[4]而导致神经元过度兴奋。阿坎酸的作用机制之一是透过正变构受体调节,增强GABAA受体的GABA讯号传导。[17][18]据称此药物能以一种新颖的方式打开氯离子通道,而不需要GABA作为辅助因子,因此比苯二氮䓬类药物更不易产生依赖性。阿坎酸已成功用于控制因鼓膜张肌痉挛而导致的耳鸣、听觉过敏、耳痛和饮酒期间的内耳压力。[21]
此外,酒精也会抑制NMDA受体的活性。[22][23]长期饮酒会导致这些受体过度生产(上调)。突然戒酒后会导致这些大量生产的NMDA受体比正常情况更为活跃,而产生震颤性谵妄和兴奋性毒性而导致神经元死亡的情况。[24]戒酒会导致谷氨酸等兴奋性神经传导物质释放激增,而过度激活NMDA受体。[25]阿坎酸可减少麸氨酸的激增。[26]该药物还表现出可保护实验室培养大鼠细胞免受乙醇戒断引起的兴奋性毒性,[27]以及受乙醇戒断加上麸氨酸暴露的影响。[28]
此药物也透过让睡眠周期第3阶段快速动眼期正常化来重建标准睡眠结构,而被认为是其药理活性的一重要作用。[20]
药物动力学
编辑阿坎酸不会被人体代谢。[18]口服阿坎酸的绝对生物利用度约为11%,[18]饮食时服用阿坎酸,其生物利用度会被降低。[29]服用的阿坎酸会以原形经由肾脏排泄。[18]
历史
编辑阿坎酸由默克集团子公司Lipha所开发。[30]于1989年获准在欧洲用于治疗酒精依赖。[31]
药业公司Forest Laboratories于2001年10月取得药物在美国的销售权。[30][32]
该药物于2004年7月获得美国食品药物管理局(FDA)核准用于医疗用途。[33]
截至2015年,名为Confluence Pharmaceuticals的药业公司在开发阿坎酸作为X染色体易裂症的治疗法。此药物在2013年被FDA授予孤儿药地位,并于2014年也被欧洲药品管理局(EMA)授予孤儿药地位。[35]
社会与文化
编辑"阿坎酸"是此药物的国际非专有药名(INN)和英国核准药名(BAN)。 "阿坎酸钙"是美国通用名(USAN)和日本接受名(JAN)。它在技术上也称为N-乙酰基高牛磺酸(N-acetylhomotaurine )或乙酰高牛磺酸(acetylhomotaurinate)。
此药物以Campral[1]及Alglutol等品牌销售。
研究
编辑此药物除能明显帮助患者戒酒之外,一些证据显示其具有神经保护作用(即它可保护神经元,免受酒精戒断以及可能的其他神经毒性原因,而造成的损伤和死亡)。[26][36]
参见
编辑- ^ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Campral label (PDF). FDA. January 2012 [2017-11-27]. (原始内容存档 (PDF)于2017-02-27). For label updates see FDA index page for NDA 021431 (页面存档备份,存于互联网档案馆)
- ^ 2.0 2.1 2.2 2.3 Plosker GL. Acamprosate: A Review of Its Use in Alcohol Dependence. Drugs. July 2015, 75 (11): 1255–1268. PMID 26084940. S2CID 19119078. doi:10.1007/s40265-015-0423-9.
- ^ Williams SH. Medications for treating alcohol dependence. American Family Physician. November 2005, 72 (9): 1775–1780 [2006-11-29]. PMID 16300039. (原始内容存档于2007-09-29).
- ^ 4.0 4.1 4.2 4.3 Malenka RC, Nestler EJ, Hyman SE, Holtzman DM. Chapter 16: Reinforcement and Addictive Disorders. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 3rd. New York: McGraw-Hill Medical. 2015. ISBN 9780071827706.
It has been hypothesized that long-term ethanol exposure alters the expression or activity of specific GABAA receptor subunits in discrete brain regions. Regardless of the underlying mechanism, ethanol-induced decreases in GABAA receptor sensitivity are believed to contribute to ethanol tolerance, and also may mediate some aspects of physical dependence on ethanol. ... Detoxification from ethanol typically involves the administration of benzodiazepines such as chlordiazepoxide, which exhibit cross-dependence with ethanol at GABAA receptors (Chapters 5 and 15). A dose that will prevent the physical symptoms associated with withdrawal from ethanol, including tachycardia, hypertension, tremor, agitation, and seizures, is given and is slowly tapered. Benzodiazepines are used because they are less reinforcing than ethanol among alcoholics. Moreover, the tapered use of a benzodiazepine with a long half-life makes the emergence of withdrawal symptoms less likely than direct withdrawal from ethanol. ... Unfortunately, acamprosate is not adequately effective for most alcoholics.
- ^ Mason BJ. Treatment of alcohol-dependent outpatients with acamprosate: a clinical review. The Journal of Clinical Psychiatry. 2001, 62 (Suppl 20): 42–48. PMID 11584875.
- ^ Nutt DJ, Rehm J. Doing it by numbers: a simple approach to reducing the harms of alcohol. Journal of Psychopharmacology. January 2014, 28 (1): 3–7. PMID 24399337. S2CID 36860967. doi:10.1177/0269881113512038.
- ^ Acamprosate. drugs.com. 2005-03-25 [2007-01-08]. (原始内容存档于2006-12-22).
- ^ Wilde MI, Wagstaff AJ. Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification. Drugs. June 1997, 53 (6): 1038–1053. PMID 9179530. S2CID 195691152. doi:10.2165/00003495-199753060-00008.
- ^ Acamprosate (Campral) Use During Pregnancy. Drugs.com. [2024-02-22]. (原始内容存档于2020-11-28) (英语).
- ^ Haber P, Lintzeris N, Proude E, Lopatko O. Guidelines for the Treatment of Alcohol Problems (PDF). Australian Government Department of Health and Ageing. [2023-02-20]. (原始内容存档 (PDF)于2022-08-20).
- ^ World Health Organization. The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. hdl:10665/371090 . WHO/MHP/HPS/EML/2023.02.
- ^ Kranzler HR, Soyka M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA. August 2018, 320 (8): 815–824. PMC 7391072 . PMID 30167705. doi:10.1001/jama.2018.11406.
- ^ Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful?. Addiction. February 2013, 108 (2): 275–293. PMC 3970823 . PMID 23075288. doi:10.1111/j.1360-0443.2012.04054.x.
- ^ Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction. June 2015, 110 (6): 920–930. PMID 25664494. doi:10.1111/add.12875.
- ^ 15.0 15.1 Saivin S, Hulot T, Chabac S, Potgieter A, Durbin P, Houin G. Clinical pharmacokinetics of acamprosate. Clinical Pharmacokinetics. November 1998, 35 (5): 331–345. PMID 9839087. S2CID 34047050. doi:10.2165/00003088-199835050-00001.
- ^ Acamprosate: Biological activity. IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. [2017-11-26]. (原始内容存档于2017-12-01).
Due to the complex nature of this drug's MMOA, and a paucity of well defined target affinity data, we do not map to a primary drug target in this instance.
- ^ 17.0 17.1 17.2 17.3 Acamprosate: Summary. IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. [2017-11-26]. (原始内容存档于2017-12-01).
Acamprosate is a NMDA glutamate receptor antagonist and a positive allosteric modulator of GABAA receptors.
Marketed formulations contain acamprosate calcium - ^ 18.0 18.1 18.2 18.3 18.4 18.5 Acamprosate. DrugBank. University of Alberta. 2017-11-19 [2017-11-26]. (原始内容存档于2017-12-01).
Acamprosate is thought to stabilize the chemical balance in the brain that would otherwise be disrupted by alcoholism, possibly by blocking glutaminergic N-methyl-D-aspartate receptors, while gamma-aminobutyric acid type A receptors are activated. ... The mechanism of action of acamprosate in the maintenance of alcohol abstinence is not completely understood. Chronic alcohol exposure is hypothesized to alter the normal balance between neuronal excitation and inhibition. in vitro and in vivo studies in animals have provided evidence to suggest acamprosate may interact with glutamate and GABA neurotransmitter systems centrally, and has led to the hypothesis that acamprosate restores this balance. It seems to inhibit NMDA receptors while activating GABA receptors.
- ^ 19.0 19.1 19.2 Kalk NJ; Lingford-Hughes AR. The clinical pharmacology of acamprosate. British Journal of Clinical Pharmacology. February 2014, 77 (2): 315–323. PMC 4014018 . PMID 23278595. doi:10.1111/bcp.12070.
- ^ 20.0 20.1 20.2 Mason BJ, Heyser CJ. Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS & Neurological Disorders Drug Targets. March 2010, 9 (1): 23–32. PMC 2853976 . PMID 20201812. doi:10.2174/187152710790966641.
- ^ Azevedo, Andréia A.; Figueiredo, Ricardo R. Tinnitus treatment with acamprosate: double-blind study. Brazilian Journal of Otorhinolaryngology. Sep-Oct 2005, 71 (5) [2024-02-04].
- ^ Malenka RC, Nestler EJ, Hyman SE. Chapter 15: Reinforcement and Addictive Disorders. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 372. ISBN 9780071481274.
- ^ Möykkynen T, Korpi ER. Acute effects of ethanol on glutamate receptors. Basic & Clinical Pharmacology & Toxicology. July 2012, 111 (1): 4–13. PMID 22429661. doi:10.1111/j.1742-7843.2012.00879.x .
- ^ Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annual Review of Medicine. 1998, 49: 173–184. PMID 9509257. doi:10.1146/annurev.med.49.1.173.
- ^ Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. The American Journal of Psychiatry. June 1998, 155 (6): 726–732 [2024-02-22]. PMID 9619143. (原始内容存档于2024-04-13).
- ^ 26.0 26.1 De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005, 19 (6): 517–537. PMID 15963001. S2CID 11563216. doi:10.2165/00023210-200519060-00004.
- ^ Mayer S, Harris BR, Gibson DA, Blanchard JA, Prendergast MA, Holley RC, Littleton J. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcoholism: Clinical and Experimental Research. October 2002, 26 (10): 1468–1478. PMID 12394279. doi:10.1097/00000374-200210000-00003.
- ^ al Qatari M, Khan S, Harris B, Littleton J. Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain. Alcoholism: Clinical and Experimental Research. September 2001, 25 (9): 1276–1283. PMID 11584146. doi:10.1111/j.1530-0277.2001.tb02348.x.
- ^ Trevor AJ. The Alcohols. Katzung BG (编). Basic & Clinical Pharmacology 14th. New York. 2017. ISBN 9781259641152. OCLC 1015240036.
- ^ 30.0 30.1 Berfield S. A CEO and His Son. Bloomberg Businessweek. 2002-05-27 [2024-02-22]. (原始内容存档于2017-12-01).
- ^ Yahn, Stephanie L.; Watterson, Lucas R. Safety and Efficacy of Acamprosate for the Treatment of Alcohol Dependence. Substance Abuse:Research and Treatment. 2013-01-31, 7 (2013) [2023-4-02-04]. doi:10.4137/SART.S9345. (原始内容存档于2023-10-20).
- ^ Press release: Forest Laboratories Announces Agreement For Alcohol Addiction Treatment. Forest Labs via Evaluate Group. 2001-10-23 [2024-02-22]. (原始内容存档于2021-08-27).
- ^ FDA Approves New Drug for Treatment of Alcoholism. FDA Talk Paper. Food and Drug Administration. 2004-07-29 [2009-08-15]. (原始内容存档于2008-01-17).
- ^ Acamprosate generics. DrugPatentWatch. [2017-11-27]. (原始内容存档于2017-12-01) (英语).
- ^ Acamprosate - Confluence Pharmaceuticals. AdisInsight. Springer Nature Switzerland AG. [2017-11-27]. (原始内容存档于2021-05-21) (英语).
- ^ Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcoholism: Clinical and Experimental Research. July 2008, 32 (7): 1105–1110. PMID 18540918. doi:10.1111/j.1530-0277.2008.00690.x.