塞浦西他啶
化合物
此條目需要精通或熟悉醫學的編者參與及協助編輯。 (2020年12月15日) |
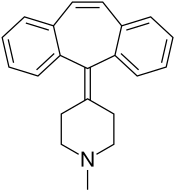
塞浦西他啶(Cyproheptadine),或名賽庚啶 ,是第一代抗組織胺藥,具有抗乙醯膽鹼和局部麻醉的功能,也是兒童抗過敏藥水希普利敏的主要成分。
 | |
 | |
| 臨床資料 | |
|---|---|
| 讀音 | (/ˌsaɪproʊˈhɛptədiːn/[1] |
| 商品名 | Periactin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682541 |
| 核准狀況 | |
| 懷孕分級 |
|
| 給藥途徑 | Oral |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 血漿蛋白結合率 | 96 to 99% |
| 藥物代謝 | Hepatic,[3][4] mostly CYP3A4 mediated. |
| 生物半衰期 | 8.6 hours[2] |
| 排泄途徑 | 糞便 (2–20%; of which, 34% as unchanged drug) 與 腎 (40%; none as unchanged drug)[3][4] |
| 識別資訊 | |
| |
| CAS號 | 129-03-3 969-33-5(鹽酸鹽) |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.482 |
| 化學資訊 | |
| 化學式 | C21H21N |
| 摩爾質量 | 287.41 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
醫療使用
編輯- 塞浦西他啶用於治療過敏反應 (特別是花粉症).[6] 用於此目的證實有效,但是第二代抗組織胺藥像是酮替酚與氯雷他定也有相同的結果,且副作用較少。[7]
- 它也被用作偏頭痛的預防性治療。在2013年的一項研究中,開始治療後7至10天內,患者的偏頭痛發生率顯著降低。這些患者在服藥前偏頭痛發作的平均頻率為每月8.7次,開始治療後3個月降至每月3.1次。[7][8] 在英國和其他一些國家/地區,藥品仿單上有此用法。
- 它也被藥品「仿單核准適應症外的使用」在嬰兒週期性嘔吐症候群的治療。這種用途的唯一證據來自回顧性研究。[9]
- 塞浦西他啶有時被「仿單核准適應症外的使用」,以改善服用抗精神病藥物的患者的靜坐不能。[10]
- 它也被藥品「仿單核准適應症外的使用」於治療各種皮膚病,包括精神性搔癢症|[11]、藥物誘發的多汗症(出汗過多)[12],以及預防表淺性單純性水疱性表皮鬆解症的水皰形成。[13]
- 該藥物的作用之一是食慾增加和體重增加,這導致該藥物在消瘦的兒童以及囊腫性纖維化患者中用於此目的(在美國為「仿單核准適應症外的使用」)。[14][15][16]
- 它也使用在「仿單核准適應症外的使用」治療中度至重度血清素綜合症,與使用血清素類藥物有關的複雜症狀,像是選擇性5-羥色胺再攝取抑制劑 (與單胺氧化酶抑制劑),以及在血清素產生的類癌腫瘤導致血液中5-羥色胺升高的案例。[17][18]
副作用
編輯- 鎮靜和睏倦(通常是短暫的)Sedation and sleepiness (often transient)
- 頭暈 Dizziness
- 協調不佳 Disturbed coordination
- 混亂 Confusion
- 躁動不安 Restlessness
- 興奮 Excitation
- 緊張 Nervousness
- 震顫 Tremor
- 易怒 Irritability
- 失眠 Insomnia
- 感覺異常 Paresthesias
- 神經炎 Neuritis
- 抽搐 Convulsions
- 欣快感 Euphoria
- 幻覺 Hallucinations
- 歇斯底里 Hysteria
- 模糊 Faintness
- 皮疹和水腫的過敏表現 Allergic manifestation of rash and edema
- 發汗 Diaphoresis
- 蕁麻疹 Urticaria
- 對光敏感 Photosensitivity
- 急性迷路炎 Acute labyrinthitis
- 複視(雙眼) Diplopia (seeing double)
- 眩暈 Vertigo
- 耳鳴 Tinnitus
- 低血壓 Hypotension (low blood pressure)
- 心悸 Palpitation
- 期前收縮 Extrasystoles
- 過敏性休克 Anaphylactic shock
- 溶血性貧血 Hemolytic anemia
- 諸如白血球減少症,粒細胞缺乏症和血小板減少症等血液異常 Blood dyscrasias such as leukopenia, agranulocytosis and thrombocytopenia
- 膽汁淤積 Cholestasis
- 對肝臟的影響:
- 上腹窘迫 Epigastric distress
- 食欲不振 Anorexia
- 噁心 Nausea
- 嘔吐 Vomiting
- 腹瀉 Diarrhea
- 抗膽鹼能副作用:
- 視力模糊 Blurred vision
- 便秘 Constipation
- 口腔乾燥症(口乾) Xerostomia (dry mouth)
- 心動過速(高心率)Tachycardia (high heart rate)
- 尿瀦留 Urinary retention
- 排尿困難 Difficulty passing urine
- 鼻塞 Nasal congestion
- 鼻或喉嚨乾燥 Nasal or throat dryness
- 頻尿 Urinary frequency
- 早期月經 Early menses
- 支氣管分泌物增厚 Thickening of bronchial secretions
- 胸悶氣喘 Tightness of chest and wheezing
- 疲勞 Fatigue
- 寒意 Chills
- 頭痛 Headache
- 食慾增加 Increased appetite
- 體重增加 Weight gain
用藥過量
編輯過量使用時,有時建議使用活性炭進行洗胃。這些症狀通常表明中樞神經系統抑制(或在某些情況下相反地刺激中樞神經系統)和過度的抗膽鹼能副作用。小鼠的半數致死量(LD50)為 123 mg/kg,,大鼠的半數致死量為 295 mg/kg 。[3][4]
藥理
編輯藥效學
編輯塞浦西他啶對此表列出的所有受體均表現為拮抗劑或逆向激動劑。[20]
| Site | Ki (nM)[a] | Action[b] | Species | Ref. |
|---|---|---|---|---|
| H1 | 0.06 | ↓ | Human | |
| H2 | ND | ND | ||
| H3 | >10,000 | Human | ||
| H4 | 202 | Human | ||
| M1 | 12 | ↓ | Human | |
| M2 | 7 | ↓ | Human | |
| M3 | 12 | ↓ | Human | |
| M4 | 8 | ↓ | Human | |
| M5 | 11.8 | ↓ | Human | |
| 5-HT1A | 59 | Human | ||
| 5-HT2A | 1.67 | ↓ | Human | |
| 5-HT2B | 1.54 | ↓ | Human | |
| 5-HT2C | 2.23 | ↓ | Human | |
| 5-HT3 | 228 | Mouse | ||
| 5-HT6 | 142 | Human | ||
| 5-HT7 | 123 | Human | ||
| D1 | 117 | Human | ||
| D2 | 112 | ↓ | Human | |
| D3 | 8 | Human | ||
| SERT | 4,100 | Rat | ||
| NET | 290 | Rat | ||
| DAT | ND | ND | ||
塞浦西他啶是一種非常有效的抗組胺藥或H1受體的拮抗劑。它在較高濃度下還具有抗膽鹼能、抗血清素能和抗多巴胺能活性。它是5-HT2受體的強效拮抗劑,這是其治療血清素綜合症的基礎。
藥物代謝動力學
編輯化學
編輯研究
編輯在一項規模較小的精神分裂症患者中,輔助使用塞浦西他啶作為輔助治療,該患者的病情穩定且正在接受其他藥物治療。雖然注意力和口語流利性似乎有所改善,但這項研究規模太小,不足以概括。[23]在另兩項針對精神分裂症患者的試驗中,也已對其進行了佐劑研究,總共約有50人,並且似乎沒有效果。[24]
獸醫用途
編輯塞浦西他啶使用在貓的食慾刺激劑[26] ,也可作為哮喘的輔助治療劑。[27] 可能的副作用包括刺激和攻擊行為。[28]它在貓的生物半衰期為 12 小時。[27]
參考資料
編輯- ^ Cyproheptadine. Dictionary.com Unabridged. Random House.
- ^ 2.0 2.1 Gunja N, Collins M, Graudins A. A comparison of the pharmacokinetics of oral and sublingual cyproheptadine. Journal of Toxicology. Clinical Toxicology. 2004, 42 (1): 79–83. PMID 15083941. S2CID 20196551. doi:10.1081/clt-120028749.
- ^ 3.0 3.1 3.2 3.3 CYPROHEPTADINE HYDROCHLORIDE tablet [Boscogen, Inc.]. DailyMed. Boscogen, Inc. November 2010 [26 October 2013]. (原始內容 (PDF)存檔於2013-07-04).
- ^ 4.0 4.1 4.2 4.3 PRODUCT INFORMATION PERIACTIN® (cyproheptadine hydrochloride) (PDF). Aspen Pharmacare Australia. Aspen Pharmacare Australia Pty Ltd. 17 November 2011 [26 October 2013]. (原始內容 (PDF)存檔於29 October 2013).
- ^ Fischer, Jnos; Ganellin, C. Robin. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 547 [2020-12-15]. ISBN 9783527607495. (原始內容存檔於2021-08-29) (英語).
- ^ MedlinePlus Drug Information: Cyproheptadine. [2020-12-15]. (原始內容存檔於2008-10-01).
- ^ 7.0 7.1 De Bruyne, P; Christiaens, T; Boussery, K; Mehuys, E; Van Winckel, M. Are antihistamines effective in children? A review of the evidence. Archives of Disease in Childhood. January 2017, 102 (1): 56–60. PMID 27335428. S2CID 21185048. doi:10.1136/archdischild-2015-310416.
- ^ Saito, Y; Yamanaka, G; Shimomura, H; Shiraishi, K; Nakazawa, T; Kato, F; Shimizu-Motohashi, Y; Sasaki, M; Maegaki, Y. Reconsideration of the diagnosis and treatment of childhood migraine: A practical review of clinical experiences. Brain & Development. May 2017, 39 (5): 386–394. PMID 27993427. S2CID 34703034. doi:10.1016/j.braindev.2016.11.011.
- ^ Salvatore, S; Barberi, S; Borrelli, O; Castellazzi, A; Di Mauro, D; Di Mauro, G; Doria, M; Francavilla, R; Landi, M; Martelli, A; Miniello, VL; Simeone, G; Verduci, E; Verga, C; Zanetti, MA; Staiano, A; SIPPS Working Group on, FGIDs. Pharmacological interventions on early functional gastrointestinal disorders. Italian Journal of Pediatrics. 16 July 2016, 42 (1): 68. PMC 4947301 . PMID 27423188. doi:10.1186/s13052-016-0272-5.
- ^ Taylor, David; Paton, Carol; Kapur, Shitij. The Maudsley Prescribing Guidelines in Psychiatry. John Wiley & Sons. 2015: 85 [2020-12-15]. ISBN 9781118754573. (原始內容存檔於2021-08-28) (英語).
- ^ Szepietowski, JC; Reszke, R. Psychogenic Itch Management 50. 2016: 124–32. ISBN 978-3-318-05888-8. PMID 27578081. doi:10.1159/000446055.
|journal=被忽略 (幫助) - ^ Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. American Journal of Psychiatry. May 2002, 159 (5): 874–5 [2020-12-15]. PMID 11986151. doi:10.1176/appi.ajp.159.5.874-a. (原始內容存檔於2012-03-19).
- ^ Pfendner, Ellen G.; Bruckner, Anna L. Epidermolysis Bullosa Simplex. GeneReviews. October 13, 2016 [2020-12-15]. PMID 20301543. (原始內容存檔於2020-10-22).
- ^ Ciproheptadina, estimulante del apetito (Cyproheptadine, appetite stimulant). [2020-12-15]. (原始內容存檔於2020-10-24).
- ^ Bioplex NF. [2020-12-15]. (原始內容存檔於2018-04-18).
- ^ Harrison ME, Norris ML, Robinson A, Spettigue W, Morrissey M, Isserlin L. Use of cyproheptadine to stimulate appetite and body weight gain: A systematic review.. Appetite. 2019, 137: 62–72. PMID 30825493. S2CID 72333631. doi:10.1016/j.appet.2019.02.012.
- ^ Rossi, S (編). Australian Medicines Handbook 2013. Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 978-0-9805790-9-3.
- ^ Iqbal, MM; Basil, MJ; Kaplan, J; Iqbal, MT. Overview of serotonin syndrome. Annals of Clinical Psychiatry. November 2012, 24 (4): 310–8. PMID 23145389.
- ^ Chertoff, Jason. Cyproheptadine-Induced Acute Liver Failure. ACG Case Reports Journal. 8 July 2014, 1 (4): 212–213. PMC 4286888 . PMID 25580444. doi:10.14309/crj.2014.56.
- ^ 20.0 20.1 Roth, BL; Driscol, J. PDSP Ki Database. Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. [14 August 2017]. (原始內容存檔於2021-08-28).
- ^ Pucci E, Petraglia F. Treatment of androgen excess in females: yesterday, today and tomorrow. Gynecol. Endocrinol. December 1997, 11 (6): 411–33. PMID 9476091. doi:10.3109/09513599709152569.
- ^ Lindsay Murray; Frank Daly; David McCoubrie; Mike Cadogan. Toxicology Handbook. Elsevier Australia. 15 January 2011: 388 [27 November 2011]. ISBN 978-0-7295-3939-5. (原始內容存檔於2014-01-01).
- ^ Buoli, M; Altamura, AC. May non-antipsychotic drugs improve cognition of schizophrenia patients?. Pharmacopsychiatry. March 2015, 48 (2): 41–50. PMID 25584772. doi:10.1055/s-0034-1396801.
- ^ 24.0 24.1 Dabaghzadeh, F; Khalili, H; Ghaeli, P; Dashti-Khavidaki, S. Potential benefits of cyproheptadine in HIV-positive patients under treatment with antiretroviral drugs including efavirenz. Expert Opinion on Pharmacotherapy. December 2012, 13 (18): 2613–24. PMID 23140169. S2CID 25769557. doi:10.1517/14656566.2012.742887.
- ^ Nunes, LV; Moreira, HC; Razzouk, D; Nunes, SO; Mari Jde, J. Strategies for the treatment of antipsychotic-induced sexual dysfunction and/or hyperprolactinemia among patients of the schizophrenia spectrum: a review. Journal of Sex & Marital Therapy. 2012, 38 (3): 281–301. PMID 22533871. S2CID 23406005. doi:10.1080/0092623X.2011.606883.
- ^ Agnew, W; Korman, R. Pharmacological appetite stimulation: rational choices in the inappetent cat. Journal of Feline Medicine and Surgery. September 2014, 16 (9): 749–56. PMID 25146662. S2CID 37126352. doi:10.1177/1098612X14545273.
- ^ 27.0 27.1 Dowling PM. Systemic Therapy of Airway Disease: Cyproheptadine. Kahn CM, Line S, Aiello SE (編). The Merck Veterinary Manual 9th. John Wiley & Sons. February 8, 2005 [2022-06-16]. ISBN 978-0-911910-50-6. (原始內容存檔於2016-03-04). Retrieved on October 26, 2008.
- ^ Dowling PM. Drugs Affecting Appetite. Kahn CM, Line S, Aiello SE (編). The Merck Veterinary Manual 9th. John Wiley & Sons. February 8, 2005 [2022-06-16]. ISBN 978-0-911910-50-6. (原始內容存檔於2012-10-28). Retrieved on October 26, 2008.
- ^ Durham, AE. Therapeutics for Equine Endocrine Disorders. The Veterinary Clinics of North America. Equine Practice. April 2017, 33 (1): 127–139. PMID 28190613. doi:10.1016/j.cveq.2016.11.003.
- ^ Merck Vet Manual. Hirsutism Associated with Adenomas of the Pars Intermedia. [April 24, 2011]. (原始內容存檔於2016-03-03).