環丙沙星
環丙沙星(INN:ciprofloxacin)是一種喹諾酮類抗生素,[4]用於治療多種病原細菌感染 - 包括骨骼和關節感染、腹腔感染、某些類型的感染性腹瀉、呼吸道感染、皮膚感染、傷寒和泌尿道感染等。[4]對於某些感染,它可與其他抗生素一起使用。[4]給藥方式有口服給藥、眼藥水、耳滴劑或是靜脈注射。[4][5]
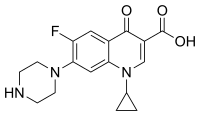 環丙沙星的化學結構 | |
 環丙沙星兩性離子三維結構 | |
| 臨床資料 | |
|---|---|
| 商品名 | Ciloxan、Cipro、Neofloxin及其他 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688016 |
| 核准狀況 | |
| 懷孕分級 | |
| 給藥途徑 | 口服給藥, 靜脈注射, 外用藥物 (耳滴劑, 眼藥水) |
| 藥物類別 | 喹諾酮類藥物 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | 70%[3] |
| 血漿蛋白結合率 | 30%[3] |
| 藥物代謝 | 肝臟 |
| 生物半衰期 | 3.5小時[3] |
| 排泄途徑 | 腎臟 |
| 識別資訊 | |
| |
| CAS號 | 85721-33-1 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.026 |
| 化學資訊 | |
| 化學式 | C17H18FN3O3 |
| 摩爾質量 | 331.35 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
使用後常見的副作用有噁心、嘔吐和腹瀉。[4]嚴重的副作用有肌腱斷裂、產生幻覺和神經損傷的風險增加。[4]重症肌無力患者的肌肉無力會惡化。[4]此藥物的副作用發生率似乎高於某些抗生素(例如頭孢菌素),但低於其他抗生素(例如克林黴素)。[6]在動物身上的研究結果,引發人們對個體於懷孕期間使用會傷害胎兒的擔憂。[7]然而少數服用該藥物的婦女所產下的嬰兒並未發現任何問題。[7]在母乳哺育期間使用似乎對於嬰兒並無安全上的顧慮。[4]此藥物是第二代喹諾酮類藥物,是種具廣譜活性的抗生素,為通常有效的殺菌藥。[4][8][9]
環丙沙星於1980年取得專利,並於1987年由拜耳公司引入醫療用途。[10][11][12]它已列入世界衛生組織基本藥物標準清單之中。[13][14]世界衛生組織(WHO)將環丙沙星列為對人類醫學不可或缺的藥物。[15]市場上有其通用名藥物流通。[4][16]此藥物於2022年在美國最常使用處方藥中排名第181,開立的處方箋數量超過200萬張。[17][18]
醫療用途
編輯環丙沙星用於治療範圍廣泛的感染,包括骨骼和關節感染、心內膜炎、細菌性腸胃炎、惡性外耳炎、腺鼠疫、呼吸道感染、蜂窩性組織炎、泌尿道感染、攝護腺炎、炭疽病和軟性下疳。[4]
環丙沙星僅可治療病原細菌感染,而不能治療普通感冒等由病毒引起的感染。對於某些病症 - 包括急性鼻竇炎、下呼吸道感染和無併發症淋病,環丙沙星並非第一線藥物。
環丙沙星在不同主要醫學會所發佈治療嚴重感染的指南中佔有重要地位,特別是那些由革蘭氏陰性菌(包括綠膿桿菌)引起的感染。例如美國傳染病學會推薦環丙沙星與甲硝唑聯合,是治療成人社區型腹部感染的幾種第一線抗生素方案之一。[19]此藥物也在治療急性腎盂腎炎、複雜性或院內感染泌尿道感染、急性或慢性攝護腺炎、[20]某些類型的心內膜炎、[21]某些皮膚感染、[22]和人工關節感感染的指南中也佔有重要地位。[23]
治療指南對其他情況的規定較為嚴格。對於較輕微的感染,通常建議優先使用較舊、作用範圍較窄的藥物作為第一線藥物,以減少產生喹諾酮類藥物抗藥性的概率。例如美國傳染病學會建議僅在已證明或預期對呋喃妥因或複方新諾明(甲氧芐啶/磺胺甲噁唑)等作用範圍較窄藥物具抗藥性的病例中改用環丙沙星和其他喹諾酮類藥物治療泌尿道感染。[24]歐洲泌尿外科學會推薦環丙沙星作為治療無併發症泌尿道感染的替代方案,但警告"必須考慮不良事件發生的可能性"。[20]
雖然監管機構批准使用環丙沙星於治療呼吸道感染,但大多數治療指南不建議採用,部分原因是其對常見呼吸道病原體肺炎鏈球菌的活性有限。[25][26][27]美國傳染病學會推薦使用治療呼吸道感染的喹諾酮類藥物,如左氧氟沙星對這種病原體具有更強的活性,作為治療有重大合併症的患者和需要住院治療的社區型肺炎的第一線藥物 。同樣的,不建議使用環丙沙星作為急性鼻竇炎的第一線藥物。[28][29]
環丙沙星在許多國家被批准用於治療淋病,但由於病菌的抗藥性已經發展,這種療法已被廣泛認為過時。[30][31][32]
懷孕
編輯專家對已發表有關懷孕期間使用環丙沙星的經驗數據進行審查,結論為懷孕期間使用的治療劑量不太可能造成重大胎兒致畸風險,但數據不足以表示風險並未存在。[33]在懷孕早期接觸包括左氧氟沙星在內的喹諾酮類藥物不會增加死產、早產、出生缺陷或低出生體重的風險。[34]
母乳哺育
編輯據報導,喹諾酮類藥物會進入母乳中,而被母乳哺育的嬰兒攝取。[35][36]
兒童
編輯由於此藥物有對肌肉骨骼系統造成永久性損傷的風險,因此美國食品藥物管理局(FDA)僅核准使用口服和靜脈注射劑於兩種適應症:
活性範圍
編輯此藥物的活性範圍包括大多數導致社區型肺炎、支氣管炎、泌尿道感染和腸胃炎的病原細菌菌株。[39]環丙沙星對革蘭氏陰性菌(如大腸桿菌、流感嗜血桿菌、肺炎克雷伯氏菌、嗜肺性退伍軍人桿菌、卡他莫拉菌、奇異變形桿菌和綠膿桿菌)特別有效,但對革蘭氏陽性菌(如對甲氧西林敏感的細菌 - 金黃色葡萄球菌、肺炎鏈球菌及糞腸球菌)的作用則較新型的喹諾酮類藥物為差。[40]
病原細菌抗藥性
編輯由於環丙沙星被廣泛用於治療本可用較舊、作用範圍較窄的抗生素即可輕易治癒的輕微感染,導致許多細菌已對其產生抗藥性,使其效力遠不如原本應有的水平。[41][42]
在治療過程中,病原細菌對環丙沙星和其他喹諾酮類藥物的抗藥性也可能會迅速發展。
迄2002年,喹諾酮類藥物已成為成人最常使用的第一類抗生素。在美國,有近一半 (42%) 的處方用於治療未經FDA批准的疾病,例如急性支氣管炎、中耳炎和急性上呼吸道感染。[43]
禁忌症
編輯使用此藥物的禁忌症有:[2]
環丙沙星也被認為禁用於兒童(上述適應症除外)、懷孕期、哺乳期母親、癲癇或其他癲癇發作的個體。
患有馬凡氏症候群或埃勒斯-當洛二氏症候群的個體須謹慎使用。[45]
不良影響
編輯不良反應可能會發生於肌腱、肌肉、關節、神經和中樞神經系統。[46][47]
此藥物不良反應的發生率似乎高於某些抗生素(例如頭孢菌素),但低於其他抗生素(例如克林黴素)。[6]一些研究發現此藥物不良反應發生率較其他抗生素為高,[48][49]而其他研究則發現並無差異。[50]
根據臨床試驗數據,大部分不良事件的嚴重性僅為輕度或中度。副作用通常在患者停止服用藥物後不久便會緩解,不需進行額外醫療介入。.[2]但有些不良影響是永久性的。[46]
肌腱問題
編輯在美國,由於環丙沙星會增加肌腱炎和肌腱斷裂的風險,特別是對於年齡超過60歲的個體、同時使用皮質類固醇的個體以及接受過腎、肺或心臟移植的個體,[51]而被附加黑框警告。肌腱斷裂可能發生在治療期間,甚至停藥後幾個月。[52]
心律不整
編輯喹諾酮類藥物(包括環丙沙星在內)會增加心臟毒性風險,導致如QT間期延長、尖端扭轉型室性心律過速、心室心律不整和心臟驟停(猝逝)。[53][47]
神經系統
編輯由於環丙沙星具有親脂性,因此能穿過血腦屏障。[54]FDA於2013年增列標籤,警告此藥物對神經系統的可能影響。
喹諾酮類藥物已被通報可導致肌陣攣,[5]尤其是環丙沙星與之有關,而衍生出"環丙陣攣(ciproclonus)"這個名詞。[8]
癌症
編輯環丙沙星在8種體外快速基因毒性篩選實驗中,有6種顯示出陽性結果,但實際在體內實驗中並未表現出基因毒性。[2]
其他
編輯另一關於環丙沙星的黑框警告是此藥物不應用於重症肌無力患者,因為可能會加劇肌無力,從而導致呼吸問題,會發生個體死亡或需使用呼吸器支持的情況。已知喹諾酮類藥物可阻斷神經肌肉傳導。有醫界人士擔心喹諾酮類藥物(包括環丙沙星在內)會影響幼兒的軟骨發育。[2]
過量
編輯過量服用環丙沙星可能會導致可逆的腎毒性。藥物過量的治療包括透過誘導嘔吐,或洗胃,以及服用含有鎂、鋁或鈣的抗酸劑以降低藥物吸收。利用血液透析或腹膜透析所排除的環丙沙星會少於10%。[55]
交互作用
編輯環丙沙星與某些食物和幾種其他藥物相互作用,會導致體內一種或兩種藥物的血清水平或分佈出現不良增加或是減少。
英國藥物安全委員會和FDA警告稱,當非類固醇抗發炎藥(NSAID)與喹諾酮類藥物聯合使用時,可能透過協同增強對γ-胺基丁酸|GABA受體的拮抗作用,而增加中樞神經系統興奮性,[2][56]中樞神經系統的不良反應(包括癲癇發作風險)會因而增加。[57][58]
作用機轉
編輯環丙沙星是一種具廣譜活性的喹諾酮類抗生素,可對一些革蘭氏陽性菌及許多革蘭氏陰性菌發生作用。[60]環丙沙星透過抑制II型拓撲異構酶(DNA旋轉酶)和IV型拓撲異構酶 ,[61][62]干擾病原細菌DNA的複製和分離,導致DNA雙股斷裂,進而抑制細菌的生長和繁殖。
藥物動力學
編輯用於全身性的環丙沙星有速釋片、緩釋片、口服混懸液及靜脈給藥溶液。環丙沙星於靜脈注射一小時後會迅速分佈到組織中,[2]某些組織中的濃度會超過血清中的濃度。對於年長及腎臟功能不佳的個體須作劑量調節。[2]
歷史
編輯日本杏林製藥株式會社(Kyorin Seiyaku Kabushiki Kaisha)於1979年提出的專利申請書中揭露其已發現具強力抗菌能力的諾氟沙星。[63]拜耳公司將諾氟沙星的結構改變,[64][65]於1983年發佈其已發明比諾氟沙星具更強抗菌效力的環丙沙星。
環丙沙星口服錠於1987年10月獲准上市,[66]僅比諾氟沙星晚一年。[67]靜脈注射劑型也1991年問世。環丙沙星的銷售額在2001年達到高峰,約為20億歐元,拜耳的專利於2004年到期,此後此藥物的年銷售額平均約為2億歐元。[68][69]
社會與文化
編輯市售配方
編輯用於全身性的環丙沙星有速釋片、緩釋片、口服混懸液和靜脈輸注溶液。它可以作為眼藥水和耳滴劑(外用藥物)。此外,環丙沙星還可與地塞米松、塞來昔布、氫羥腎上腺皮質素和醋酸氟輕松等藥物組成複方藥。[70]
研究
編輯隨着環丙沙星受到廣泛使用,許多病原細菌已對其產生抗藥性。科學家們為應對這種問題均致力開發新的抗生素,這些新藥物不僅能有效對抗已產生抗藥性的細菌,甚至也顯示出在治療病毒感染方面的潛力。[71]
參考文獻
編輯- ^ Ciprofloxacin Use During Pregnancy. Drugs.com. 2019-01-07 [2019-12-19].
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Cipro- ciprofloxacin hydrochloride tablet, film coated; Cipro- ciprofloxacin kit. DailyMed. 2023-01-31 [2024-02-09].
- ^ 3.0 3.1 3.2 Zhanel GG, Fontaine S, Adam H, Schurek K, Mayer M, Noreddin AM, Gin AS, Rubinstein E, Hoban DJ. A Review of New Fluoroquinolones: Focus on their Use in Respiratory Tract Infections. Treatments in Respiratory Medicine. 2006, 5 (6): 437–465. PMID 17154673. S2CID 26955572. doi:10.2165/00151829-200605060-00009.
- ^ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 Ciprofloxacin Hydrochloride. The American Society of Health-System Pharmacists. [2015-08-23]. (原始內容存檔於2015-09-23).
- ^ 5.0 5.1 Ciprofloxacin Hcl Drops. WebMD. 2018-02-22 [2018-02-22].
- ^ 6.0 6.1 Heidelbaugh JJ, Holmstrom H. The perils of prescribing fluoroquinolones. The Journal of Family Practice. April 2013, 62 (4): 191–197. PMID 23570031.
- ^ 7.0 7.1 Prescribing medicines in pregnancy database. Government of Australia. 2015-08-23. (原始內容存檔於2014-04-08).
- ^ 8.0 8.1 Ball P. Quinolone generations: natural history or natural selection?. The Journal of Antimicrobial Chemotherapy. July 2000,. 46 Suppl T1: 17–24. PMID 10997595. doi:10.1093/oxfordjournals.jac.a020889 .
- ^ Oliphant CM, Green GM. Quinolones: a comprehensive review. American Family Physician. February 2002, 65 (3): 455–464. PMID 1185862. doi:10.1016/s0022-5347(17)67120-9.
- ^ Oxford Handbook of Infectious Diseases and Microbiology. OUP Oxford. 2009: 56. ISBN 978-0-19-103962-1. (原始內容存檔於2017-09-08).
- ^ Fischer J, Ganellin CR. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 500. ISBN 978-3-527-60749-5.
- ^ Contemporary Chemical Approaches for Green and Sustainable Drugs,2022. Elsevier. [2024-11-22]. ISBN 978-0-12-822248-5.
- ^ World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. hdl:10665/325771 . WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization. World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. hdl:10665/345533 . WHO/MHP/HPS/EML/2021.02.
- ^ World Health Organization. Critically important antimicrobials for human medicine 6th revision. Geneva: World Health Organization. 2019. ISBN 978-92-4-151552-8. hdl:10665/312266 .
- ^ Hamilton RJ. Tarascon pharmacopoeia 15th. Jones & Bartlett Publishers. 2014: 85. ISBN 978-1-284-05671-6. (原始內容存檔於2017-09-08).
- ^ The Top 300 of 2022. ClinCalc. [2024-08-30]. (原始內容存檔於2024-08-30).
- ^ Ciprofloxacin Drug Usage Statistics, United States, 2013 - 2022. ClinCalc. [2024-08-30].
- ^ Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clinical Infectious Diseases. January 2010, 50 (2): 133–64. PMID 20034345. doi:10.1086/649554 .
- ^ 20.0 20.1 Grabe M, Bjerklund-Johansen TE, Botto H, Çek M, Naber KG, Pickard RS, Tenke P, Wagenlehner F, Wullt B. Guidelines on Urological Infections (PDF). European Association of Urology. 2013. (原始內容 (PDF)存檔於2013-12-31).
- ^ Baddour LM, Wilson WR, Bayer AS, Fowler VG, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. June 2005, 111 (23): e394–434. PMID 15956145. doi:10.1161/CIRCULATIONAHA.105.165564 .
- ^ Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan EL, Montoya JG, Wade JC. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clinical Infectious Diseases. November 2005, 41 (10): 1373–406. PMID 16231249. doi:10.1086/497143 .
- ^ Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases. January 2013, 56 (1): e1–e25. PMID 23223583. doi:10.1093/cid/cis803 .
- ^ Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinical Infectious Diseases. March 2011, 52 (5): e103–20. PMID 21292654. doi:10.1093/cid/ciq257 .
- ^ Hoogkamp-Korstanje JA, Klein SJ. Ciprofloxacin in acute exacerbations of chronic bronchitis. The Journal of Antimicrobial Chemotherapy. September 1986, 18 (3): 407–413. PMID 3490468. doi:10.1093/jac/18.3.407.
- ^ Vardakas KZ, Siempos II, Grammatikos A, Athanassa Z, Korbila IP, Falagas ME. Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials. Canadian Medical Association Journal. December 2008, 179 (12): 1269–1277. PMC 2585120 . PMID 19047608. doi:10.1503/cmaj.080358.
- ^ Donaldson PM, Pallett AP, Carroll MP. Ciprofloxacin in general practice. BMJ. May 1994, 308 (6941): 1437. PMC 2540361 . PMID 8019264. doi:10.1136/bmj.308.6941.1437.
- ^ Karageorgopoulos DE, Giannopoulou KP, Grammatikos AP, Dimopoulos G, Falagas ME. Fluoroquinolones compared with beta-lactam antibiotics for the treatment of acute bacterial sinusitis: a meta-analysis of randomized controlled trials. Canadian Medical Association Journal. March 2008, 178 (7): 845–854. PMC 2267830 . PMID 18362380. doi:10.1503/cmaj.071157.
- ^ Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, Pankey GA, Seleznick M, Volturo G, Wald ER, File TM. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clinical Infectious Diseases. April 2012, 54 (8): e72–e112. PMID 22438350. S2CID 1946193. doi:10.1093/cid/cir1043 .
- ^ Gonococcal Isolate Surveillance Project (GISP) Annual Report – 2003 (PDF). U.S. Centers for Disease Control and Prevention (CDC). November 2004 [2009-08-31]. (原始內容存檔 (PDF)於2009-04-24).
- ^ Young H, Palmer J, Winter A. Ciprofloxacin resistant gonorrhoea: the situation in Scotland and implications for therapy (PDF). SCIEH Weekly Report. 2003-07-22, 37 [2009-08-30]. ISSN 1357-4493. (原始內容 (PDF)存檔於2011-07-22).
- ^ Centers for Disease Control and Prevention (CDC). Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections (PDF). MMWR. Morbidity and Mortality Weekly Report. April 2007, 56 (14): 332–336. PMID 17431378.
- ^ Barolin GS. [Illness, anxiety and the physician. An example from neurology and neurorehabilitation]. Wiener Medizinische Wochenschrift. May 1995, 141 (22): 512–25. PMC 1801454 . PMID 1801454.
- ^ Ziv A, Masarwa R, Perlman A, Ziv D, Matok I. Pregnancy Outcomes Following Exposure to Quinolone Antibiotics – a Systematic-Review and Meta-Analysis. Pharm. Res. March 2018, 35 (5): 109. PMID 29582196. S2CID 4724821. doi:10.1007/s11095-018-2383-8.
- ^ Shin HC, Kim JC, Chung MK, Jung YH, Kim JS, Lee MK, Amidon GL. Fetal and maternal tissue distribution of the new fluoroquinolone DW-116 in pregnant rats. Comparative Biochemistry and Physiology. Toxicology & Pharmacology. September 2003, 136 (1): 95–102. PMID 14522602. doi:10.1016/j.cca.2003.08.004.
- ^ Dan M, Weidekamm E, Sagiv R, Portmann R, Zakut H. Penetration of fleroxacin into breast milk and pharmacokinetics in lactating women. Antimicrobial Agents and Chemotherapy. February 1993, 37 (2): 293–6. PMC 187655 . PMID 8452360. doi:10.1128/AAC.37.2.293 .
- ^ Murphy D. Cipro Labeling Revision Letter 08/30/2000 Supplement 008 New or Modified Indication (PDF). U.S. Food and Drug Administration. 2000-08-30. (原始內容存檔 (PDF)於2012-10-18).
- ^ Albrecht R. Cipro Labeling Revision Letter 03/25/2004 Supplement 049 Patient Population Altered (PDF). U.S. Food and Drug Administration. 2004-03-25 [2009-09-07]. (原始內容存檔 (PDF)於2012-10-18).
- ^ Johannsen EC, Sabatine MS. Pharmcards review cards for medical students 4th. Philadelphia: Wolters Kluwer|Lippincott Williams & Wilkins. 2010. ISBN 978-0-7817-8741-3. OCLC 893525059.[頁碼請求]
- ^ Hooper D. Fluoroquinolones. UpToDate. 2018-02-12 [2018-02-26].
- ^ Vatopoulos AC, Kalapothaki V, Legakis NJ. Bacterial resistance to ciprofloxacin in Greece: results from the National Electronic Surveillance System. Greek Network for the Surveillance of Antimicrobial Resistance. Emerging Infectious Diseases. 1999, 5 (3): 471–6. PMC 2640758 . PMID 10341191. doi:10.3201/eid0503.990325.
- ^ Bacterial resistance prompts concern among health officials. Minnesota Department of Health. 2009-02-26. (原始內容存檔於2009-03-05).
- ^ Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. The American Journal of Medicine. March 2005, 118 (3): 259–68. PMID 15745724. doi:10.1016/j.amjmed.2004.09.015.
- ^ Thai T, Salisbury BH, Zito PM. Ciprofloxacin. StatPearls. Treasure Island, FL: StatPearls Publishing. 2022 [2022-01-31]. PMID 30571075.
- ^ LeMaire SA, Zhang L, Zhang NS, Luo W, Barrish JP, Zhang Q, Coselli JS, Shen YH. Ciprofloxacin accelerates aortic enlargement and promotes dissection and rupture in Marfan mice. The Journal of Thoracic and Cardiovascular Surgery. March 2022, 163 (3): e215–e226. PMID 34586071. S2CID 224937717. doi:10.1016/j.jtcvs.2020.09.069 .
- ^ 46.0 46.1 Drug Safety and Availability – FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. U.S. Food and Drug Administration (FDA). [2018-01-10].
- ^ 47.0 47.1 Liu X, Ma J, Huang L, Zhu W. Fluoroquinolones increase the risk of serious arrhythmias: A systematic review and meta-analysis. Medicine (Baltimore). November 2017, 96 (44): e8273. PMC 5682775 . PMID 29095256. doi:10.1097/MD.0000000000008273.
- ^ Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrobial Agents and Chemotherapy. May 2013, 57 (5): 2326–32. PMC 3632900 . PMID 23478961. doi:10.1128/AAC.02176-12.
- ^ Falagas ME, Matthaiou DK, Vardakas KZ. Fluoroquinolones vs beta-lactams for empirical treatment of immunocompetent patients with skin and soft tissue infections: a meta-analysis of randomized controlled trials. Mayo Clinic Proceedings. December 2006, 81 (12): 1553–66. PMID 17165634. doi:10.4065/81.12.1553.
- ^ Knottnerus BJ, Grigoryan L, Geerlings SE, Moll van Charante EP, Verheij TJ, Kessels AG, ter Riet G. Comparative effectiveness of antibiotics for uncomplicated urinary tract infections: network meta-analysis of randomized trials. Family Practice. December 2012, 29 (6): 659–70. PMID 22516128. doi:10.1093/fampra/cms029 .
- ^ Stephenson AL, Wu W, Cortes D, Rochon PA. Tendon Injury and Fluoroquinolone Use: A Systematic Review. Drug Saf. September 2013, 36 (9): 709–21. PMID 23888427. S2CID 24948660. doi:10.1007/s40264-013-0089-8.
- ^ Saint F, Gueguen G, Biserte J, Fontaine C, Mazeman E. [Rupture of the patellar ligament one month after treatment with fluoroquinolone] [Rupture of the patellar ligament one month after treatment with fluoroquinolone]. Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur. September 2000, 86 (5): 495–7. PMID 10970974 (法語).
- ^ Gorelik E, Masarwa R, Perlman A, Rotshild V, Abbasi M, Muszkat M, Matok I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-analysis and Network Meta-analysis. Drug Saf. October 2018, 42 (4): 529–538. PMID 30368737. S2CID 53105534. doi:10.1007/s40264-018-0751-2.
- ^ Babar SM. SIADH associated with ciprofloxacin. The Annals of Pharmacotherapy. October 2013, 47 (10): 1359–63. PMID 24259701. S2CID 36759747. doi:10.1177/1060028013502457.
- ^ Cipro Labeling Revision 04/06/2009 Supplement 073 (PDF). U.S. Food and Drug Administration (FDA). 2009-04-06 [2009-09-08]. (原始內容存檔 (PDF)於2010-07-05).
- ^ Royal Pharmaceutical Society of Great Britain. 5 Infections. British National Formulary (BNF 57). BMJ Group and RPS Publishing. 2009. ISBN 978-0-85369-845-6.
- ^ De Sarro A, De Sarro G. Adverse reactions to fluoroquinolones. an overview on mechanistic aspects. Current Medicinal Chemistry. March 2001, 8 (4): 371–84. PMID 11172695. doi:10.2174/0929867013373435.
- ^ Brouwers JR. Drug interactions with quinolone antibacterials. Drug Safety. 1992, 7 (4): 268–81. PMID 1524699. S2CID 6701544. doi:10.2165/00002018-199207040-00003.
- ^ Haddad A, Davis M, Lagman R. The pharmacological importance of cytochrome CYP3A4 in the palliation of symptoms: review and recommendations for avoiding adverse drug interactions. Supportive Care in Cancer. March 2007, 15 (3): 251–7. PMID 17139496. S2CID 9186457. doi:10.1007/s00520-006-0127-5.
- ^ First aid for the USMLE step 2 CK 6th. McGraw-Hill Medical. June 2007. ISBN 978-0-07-148795-5.
- ^ Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiology and Molecular Biology Reviews. September 1997, 61 (3): 377–92. PMC 232616 . PMID 9293187. doi:10.1128/mmbr.61.3.377-392.1997.
- ^ Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chemistry & Biology //www.ncbi.nlm.nih.gov/pmc/articles/PMC7316379
|PMC=缺少標題 (幫助). May 2010, 17 (5): 421–33. PMC 7316379 . PMID 20534341. doi:10.1016/j.chembiol.2010.04.012 . - ^ Khan MY, Gruninger RP, Nelson SM, Klicker RE. Comparative in vitro activity of norfloxacin (MK-0366) and ten other oral antimicrobial agents against urinary bacterial isolates. Antimicrobial Agents and Chemotherapy. May 1982, 21 (5): 848–51. PMC 182027 . PMID 6213200. doi:10.1128/AAC.21.5.848.
- ^ Patent US4547503 – 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[4-(oxo-alkyl)-1-piperazinyl ... – Google Patents.
- ^ Patent US4544658 – 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(alkyl-1-piperazinyl)quinoline-3 ... – Google Patents.
- ^ Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations N019537. U.S. Food and Drug Administration (FDA). [2014-01-05]. (原始內容存檔於2014-01-06).
- ^ Orange Book Detail Record Search. U.S. Food and Drug Administration (FDA). (原始內容存檔於2014-01-06).
- ^ www.sec.gov. (原始內容存檔於2017-07-09).
- ^ Dan Prochilo for Law360 2013-11-18 Bayer's $74M Cipro Pay-For-Delay Deal Approved In Calif. 互聯網檔案館的存檔,存檔日期2015-03-18.
- ^ Otovel (- ciprofloxacin and fluocinolone acetonide solution. DailyMed. 2019-09-12 [2024-02-09].
- ^ Zhang GF, Liu X, Zhang S, Pan B, Liu ML. Ciprofloxacin derivatives and their antibacterial activities. European Journal of Medicinal Chemistry. February 2018, 146: 599–612. PMID 29407984. doi:10.1016/j.ejmech.2018.01.078.
外部連結
編輯- Ciprofloxacin Ophthalmic. MedlinePlus.