多西环素
此条目需要扩充。 (2010年9月19日) |
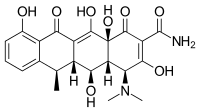
独克士霉素(INN:doxycycline,也称为多喜霉素、强力霉素、伟霸霉素、脱氧土霉素或去氧土霉素)是种广谱四环素类抗生素,用于治疗病原细菌和某些寄生虫的感染[1] - 如细菌性肺炎、痤疮、披衣菌感染、莱姆病、霍乱、斑疹伤寒和梅毒,[1]也可用于预防疟疾。[2][3]
 | |
 | |
| 临床资料 | |
|---|---|
| 读音 | /ˌdɒksɪˈsaɪkliːn/ DOKS-iss-EYE-kleen |
| 商品名 | Doxy、Doryx、Vibramycin及其他 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682063 |
| 核准状况 | |
| 怀孕分级 |
|
| 给药途径 | 口服给药, 静脉注射[1] |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 生物利用度 | ~100% |
| 血浆蛋白结合率 | 80–90% |
| 药物代谢 | 微小,可忽略不计 |
| 生物半衰期 | 10–22小时 |
| 排泄途径 | 多数透过粪便, 40%透过尿液 |
| 识别信息 | |
| |
| CAS号 | 564-25-0 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.429 |
| 化学信息 | |
| 化学式 | C22H24N2O8 |
| 摩尔质量 | 444.44 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
使用后常见的副作用有腹泻、恶心、呕吐、腹痛以及晒伤风险增加。[1]不建议个体在怀孕期间使用,以免对胎儿造成伤害。[1]此药物与其他四环素类抗生素药剂一样,系透过抑制病菌蛋白质的生成而减缓其繁殖或是将其杀死。[1][4]它透过靶向疟原虫的色素体胞器(顶质体),而将其杀死。[5][6]
独克士霉素于1957年取得专利,并于1967年由辉瑞制药取得医疗用途核准。[7][8]它已纳入世界卫生组织基本药物标准清单之中。[9]市面上有其通用名药物流通。[1][10]此药物在美国2022年最常使用处方药中排名第68,开立的处方笺数量超过900万张。[11][12]
医疗用途
编辑独克士霉素除能用于四环素类抗生素所有成员的一般适应症外,也常用于治疗莱姆病、慢性前列腺炎、鼻窦炎、骨盆腔发炎、[13][14]严重痤疮、酒糟鼻、[15][16][17]和立克次体感染。[18]口服此药物以治疗丘疹脓疱性酒糟鼻和成人痤疮的药理作用不仅限于其抗菌活性,它的抗炎作用和对血管生成抑制作用也是疗效的重点。[19]
此药物于2004年在加拿大被认为是治疗披衣菌感染和非淋菌性尿道炎的一线药物,并与头孢克肟联合用于治疗无并发症的淋病。[20]
抗菌性
编辑一般适应症
编辑独克士霉素是一种广谱抗生素,用于治疗多种细菌感染。对卡他莫拉氏菌、羊布鲁氏菌、肺炎披衣菌、肺炎支原体等病菌能发挥作用。此外此药物也用于预防和治疗炭疽、钩端螺旋体病、腺鼠疫和莱姆病等严重疾病。然而一些细菌,包括嗜血杆菌属、人类支原体和绿脓杆菌,已出现对独克士霉素的抗药性。[21][22]此药物也能有效对抗鼠疫耶尔森菌(腺鼠疫的传染源),并用于治疗莱姆病、[23][24][25][26]埃利希体病、[27][28]和落矶山斑点热。[29]
- 落矶山斑点热、斑疹伤寒和斑疹伤寒群(一组由立克次体引起的传染病)、恙虫病、[31]Q型流感、[32]立克次氏体痘和由立克次体引起的蜱热、[33][34][35]
- 肺炎枝原体引起的呼吸道感染、[36]
- 花柳性淋巴肉芽肿、沙眼、结膜炎及砂眼衣原体导致的成人单纯性尿道、子宫颈内膜或直肠感染、[29][30]
- 鹦鹉热,[29][30]
- 解脲脲原体引起的非淋菌性尿道炎、[29][30]
- 伯氏疏螺旋体引起的回归热、[29][30]
- 杜克雷嗜血杆菌引起的软性下疳、[29][30]
- 鼠疫耶尔森菌引起的腺鼠疫、[29][30]
- 兔热病、[29][30]
- 霍乱、[29][30]
- 弯曲杆菌胎儿型感染、[29][30]
- 布鲁氏菌属引起的布鲁氏菌病(与链霉素联合使用)、[29][30]
- 巴尔通体病、[29][30]
- 腹股沟肉芽肿(克雷伯氏菌属)、[29][30]
- 莱姆病:[37]可用于成人及儿童,且适用于各年龄层儿童的治疗或预防,最长治疗周期为21天。[38]
特定革兰氏阴性菌适应症
编辑若细菌学检测证实感染病原为对独克士霉素敏感的革兰氏阴性菌,则可选用此药物进行治疗:[29][30]
- 大肠杆菌感染、[29][30]
- 产气肠杆菌感染、[29][30]
- 志贺氏菌属感染、[29][30]
- 不动杆菌属感染、[29][30]
- 流感嗜血杆菌引起的呼吸道感染、[29][30]
- 克雷伯氏菌属引起的呼吸道感染和泌尿道感染。[29][30]
特定革兰氏阳性菌适应症
编辑一些革兰氏阳性菌已对独克士霉素产生抗药性。高达44%的化脓性链球菌和高达74%的粪链球菌属已具抗药性。高达57%的痤疮丙酸杆菌菌株已具抗药性。[39]若细菌学检测证实感染病原为对独克士霉素敏感的革兰氏阳性菌,则可选用此药物进行治疗:[29][30]
当病人禁用青霉素时
编辑当个体不能施用青霉素时,可使用独克士霉素治疗:[29][30]
- 由梅毒密螺旋体引起的梅毒、[29][30]
- 由雅司螺旋体引起的热带肉芽肿、[29][30]
- 由单核细胞增生李斯特菌引起的李斯特菌病、[29][30]
- 由梭杆菌属引起的坏死性牙龈炎、[29][30]
- 由衣氏放线菌引起的放线菌病、[29][30]
- 由梭菌属引起的感染。[29][30]
用作辅助制剂
编辑独克士霉素也可用作严重痤疮的辅助制剂。[40][29][30]
亚抗菌剂量独克士霉素 (SDD) 广泛用作牙周炎刮治和根面平整术的辅助用药。SDD也用于治疗痤疮和酒糟鼻等皮肤病,[15][41][42]包括眼部红斑痤疮。
此药物也用作软性下疳的辅助制剂。[43]
作为性传染病预防用途
编辑此药物可用于暴露后预防(PEP),以减少性传染病感染(STI)的发生率,但它与相关的四环素抗药性有关联,特别是淋球菌。[44][45][46]澳大利亚共识声明因而提到基于抗生素耐药性的考量,独克士霉素在暴露后预防中的应用应予谨慎。目前证据显示此药物对于男男性行为者梅毒的预防效果较佳,但对于其他细菌性性传染病感染,其益处与风险的比值尚不明确。[47]
美国疾病管制与预防中心 (CDC)[48]和澳大利亚爱滋病医学协会的指引支持在PEP中适当使用独克士霉素。[49][50]
组合使用
编辑治疗布鲁氏菌病的一线药物是独克士霉素和链霉素的组合,二线的是独克士霉素和利福平的组合。[51]
抗疟药
编辑独克士霉素对恶性疟原虫的红血球阶段(erythrocytic stage)具有活性,但对恶性疟原虫的配子母细胞没作用。[52]此药物可用于预防疟疾。不建议单独用于疟疾的初始治疗,虽然寄生虫会对此药物的效力敏感,但其抗疟作用会延迟发生,疟原虫在此期间可能继续繁殖,有可能导致病情恶化。。[53]
独克士霉素会阻断恶性疟原虫顶质体(细胞器)中的蛋白质产生,而发生两重要作用:破坏寄生虫产生对其生长非常重要的脂肪酸能力,且损害疟原虫血红素(一种辅助因子)的产生。这些影响发生在寄生虫生命周期的后期,对寄生虫的抑制作用主要发生在血液期,此阶段是疟原虫引起宿主发病的主因。[54]独克士霉素透过此过程,既能抑制恶性疟原虫的生长,又能防止其繁殖。它不会直接杀死恶性疟原虫,而是创造阻止其生长和复制的条件。[55]
世界卫生组织 (WHO) 发布的指南指出,独克士霉素与青蒿琥酯或是奎宁组合后,可用于治疗恶性疟原虫引起的无并发症疟疾,或作为严重疟疾静脉注射治疗后的续接疗法。[56]
驱虫药
编辑独克士霉素可杀死线虫生殖道中的共生沃尔巴克氏体属细菌,导致线虫不孕,而减少蟠尾丝虫症和淋巴丝虫病等疾病的传播。[57]于2005年进行的现场试验,显示为期8周的独克士霉素疗程几乎将微丝蚴的释放清除。[58]
硬化剂注射疗法
编辑仿单标示外使用
编辑独克士霉素可用于仿单标示外用途,以治疗家族性淀粉样物多发性神经病变 (ATTR)。研究显示独克士霉素与熊去氧胆酸的联合疗法,可有效破坏ATTR患者体内已形成的转甲状腺素蛋白纤维,为治疗此病提供新的希望。[60]
给药途径
编辑独克士霉素可透过口服或是静脉注射途径给药。[1]
此药物与乳制品、抗酸剂、钙补充剂、含铁制剂、含镁泻药或胆汁酸螯合剂一同服用并不危险,但这些食物和补充剂都可能会降低人体对独克士霉素的吸收率。[61][62]
禁忌症
编辑有严重肝病或同时使用异维A酸或其他类视黄醇(与维生素A相似的有机化合物)禁用此药物,因为在极少数情况下,四环素和类视黄醇均会导致高颅内压。[61]
怀孕与哺乳
编辑独克士霉素被FDA归类为D级妊娠药物(风险证据明确:已有明确证据显示对人类胎儿有风险)。它会进入母乳。[63]其他四环素抗生素于个体在怀孕期间和8岁以下儿童禁用,因为它们可能会破坏骨骼和牙齿的发育。[64]
不良反应
编辑使用此药物的通常不良反应有头痛、恶心、呕吐及皮肤对阳光敏感。[65] 严重副作用属于罕见(少于0.1%):不明原因的流血,包括流鼻血(可能是血液问题)、腹泻且便中有血或是粘液、耳鸣、浅色排便及深色尿液(可能是肝脏问题)、用药后开始关节痛或肌肉痛、剧烈头痛连同呕吐及视力问题以及光照性指甲剥离等。[65]
药物交互作用
编辑先前人们认为独克士霉素会加速细胞色素P450(一个庞大的酶蛋白质超家族)分解避孕药,而损害多种激素避孕药的有效性。研究显示使用大多数四环素类抗生素(包括独克士霉素)时,口服避孕药的有效性并无明显下降,但许多医生仍然建议服用此药物者另采屏障避孕法以防意外怀孕。[66][67][68]
药理学
编辑独克士霉素与其他四环素抗生素同样具有抑菌作用 - 透过抑制病菌的蛋白质合成来防止其繁殖。[69]
作用机转
编辑独克士霉素是一种广谱抑菌抗生素。它透过与仅存在于病菌中的30S核糖体亚基结合来抑制其蛋白质合成。[70][71]而阻止转运RNA与核糖体亚基上的信使RNA结合,表示胺基酸无法添加到多肽链中,也无法产生新的蛋白质,而阻止病菌生长,让人体免疫系统有时间杀死和清除病菌。[72]
药物动力学
编辑药物几乎完全经由服用者的小肠上部吸收。它在血浆中的最大血药浓度于给药后一到两小时内发生。血浆蛋白结合率高达近80-90%。
独克士霉素在人体的代谢微小,可忽略不计。经人体主动排泄到肠道(部分通过胆囊,部分经由血管),其中一些会形成螯合物而失去活性。有约40%经由肾脏消除,
历史
编辑由于青霉素出现,在第二次世界大战期间彻底改变病菌感染的治疗方法,许多药厂开始利用生物勘探以发现抗生素的模式。美国氰胺制药(于2009年为辉瑞制药并入)为其中之一,该公司的研究人员在1940年代末期发现金霉素,此为四环素类抗生素的第一种。[4]
随后辉瑞制药发明土霉素及四环霉素,最终制造出独克士霉素,并于1960年代初进行临床开发,于1967年获得FDA批准用于医疗用途。[4]
当独克士霉素的专利在1970年代初接近到期时,此专利成为辉瑞制药和国际整流器公司[73]之间诉讼的主题,最终是辉瑞制药胜诉。争端直到1983年才落幕。[74]
FDA于2013年1月报告称,"由于需求增加和制造问题" ,部分(并非全部)独克士霉素出现短缺现象。[75]导致美国独克士霉素的市场价格于2013年和2014年初大幅上涨(从每瓶500片的20美元上涨到超过1,800美元),[76][77][78]过后才下降。.[79][80]
社会与文化
编辑研究
编辑有研究以此药物应用于下述疾病的治疗:
抗发炎剂
编辑一些研究显示独克士霉素是一可透过抑制促炎性细胞因子来发挥抗发炎特性的潜在药剂。[84]
伤口愈合
编辑科学家们于近年积极投入研究,以开发出更有效的独克士霉素,加速伤口愈合。研究的重点主要放在两方面:
- 延长药物保存期限
- 改善药物投递方式
最常见的投递方式是伤口敷料。相较于过去单层的敷料,最新的设计已发展出三层结构,这种设计能更有效地将药物释放到伤口,促进愈合。[85]
研究试剂
编辑独克士霉素和其他四环素类抗生素常被用作in vitro(体外)和in vivo(体内)生物医学研究实验中的研究试剂。
- 涉及细菌的实验: 独克士霉素和其他四环素类抗生素常被用于研究细菌相关的生物医学实验。
- 涉及真核细胞的实验: 在研究真核细胞时,四环素系统常被用来控制基因的表达。研究人员借由独克士霉素可精确控制目标蛋白的产生时间和数量。
参考文献
编辑- ^ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 Doxycycline calcium. The American Society of Health-System Pharmacists. [2015-08-18]. (原始内容存档于2015-09-23).
- ^ Malaria. Fit for Travel. Public Health Scotland. Chemoprophylaxis. [2023-12-04]. (原始内容存档于2023-12-04).
- ^ Choosing a Drug to Prevent Malaria. Centers for Disease Control and Prevention. Doxycycline. February 2023 [2023-12-04]. (原始内容存档于2016-11-13).
- ^ 4.0 4.1 4.2 Nelson ML, Levy SB. The history of the tetracyclines. Annals of the New York Academy of Sciences. December 2011, 1241 (1): 17–32. Bibcode:2011NYASA1241...17N. PMID 22191524. S2CID 34647314. doi:10.1111/j.1749-6632.2011.06354.x.
- ^ McFadden GI. Apicoplast. Current Biology. March 2014, 24 (7): R262–3. Bibcode:2014CBio...24.R262M. PMID 24698369. doi:10.1016/j.cub.2014.01.024 .
- ^ Schlagenhauf-Lawlor P. Travelers' Malaria. PMPH-USA. 2008: 148. ISBN 978-1-55009-336-0.
- ^ Fischer J, Ganellin CR. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 489. ISBN 978-3-527-60749-5.
- ^ Corey EJ. Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. 2013: 406 [2017-09-09]. ISBN 978-1-118-35446-9. (原始内容存档于2023-01-14).
- ^ World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ 10.0 10.1 Hamilton RJ. Tarascon pharmacopoeia 12th. Sudbury, MA: Jones & Bartlett Learning. 2011: 79. ISBN 978-1-4496-0067-9.
- ^ The Top 300 of 2022. ClinCalc. [2024-08-30]. (原始内容存档于2024-08-30).
- ^ Doxycycline Drug Usage Statistics, United States, 2013 - 2022. ClinCalc. [2024-08-30]. (原始内容存档于2020-07-08).
- ^ Sweet RL, Schachter J, Landers DV, Ohm-Smith M, Robbie MO. Treatment of hospitalized patients with acute pelvic inflammatory disease: comparison of cefotetan plus doxycycline and cefoxitin plus doxycycline. American Journal of Obstetrics and Gynecology. March 1988, 158 (3 Pt 2): 736–41. PMID 3162653. doi:10.1016/S0002-9378(16)44537-0.
- ^ Gjønnaess H, Holten E. Doxycycline (Vibramycin) in pelvic inflammatory disease. Acta Obstetricia et Gynecologica Scandinavica. 1978, 57 (2): 137–9. PMID 345730. S2CID 28328073. doi:10.3109/00016347809155893.
- ^ 15.0 15.1 Holmes NE, Charles PG. Safety and Efficacy Review of Doxycycline. Clinical Medicine. Therapeutics. 5 January 2009, 1: CMT.S2035. S2CID 58790579. doi:10.4137/CMT.S2035.
- ^ Määttä M, Kari O, Tervahartiala T, Peltonen S, Kari M, Saari M, Sorsa T. Tear fluid levels of MMP-8 are elevated in ocular rosacea--treatment effect of oral doxycycline. Graefe's Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie. August 2006, 244 (8): 957–62. PMID 16411105. S2CID 20540747. doi:10.1007/s00417-005-0212-3.
- ^ Quarterman MJ, Johnson DW, Abele DC, Lesher JL, Hull DS, Davis LS. Ocular rosacea. Signs, symptoms, and tear studies before and after treatment with doxycycline. Archives of Dermatology. January 1997, 133 (1): 49–54. PMID 9006372. doi:10.1001/archderm.133.1.49.
- ^ Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. The Medical Clinics of North America. November 2008, 92 (6): 1345–61, x. PMID 19061755. doi:10.1016/j.mcna.2008.06.002.
- ^ Lee JJ, Chien AL. Rosacea in Older Adults and Pharmacologic Treatments. Drugs Aging. April 2024, 41 (5): 407–421. PMID 38649625. doi:10.1007/s40266-024-01115-y.
- ^ Rekart ML. Doxycycline:" New" treatment of choice for genital chlamydia infections.. British Columbia Medical Journal. December 2004, 46 (10): 503. (原始内容存档于2017-02-02).
- ^ Doxycycline spectrum of bacterial susceptibility and Resistance (PDF). [2012-05-04]. (原始内容 (PDF)存档于2014-02-01).
- ^ Stoddard RA, Galloway RL, Guerra MA. Leptospirosis. Yellow Book. Atlanta, GA: Centers for Disease Control and Prevention. 2015-07-10 [2017-04-16]. (原始内容存档于2017-04-09).
- ^ Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Annals of Internal Medicine. August 1992, 117 (4): 273–80. PMID 1637021. S2CID 23358315. doi:10.7326/0003-4819-117-4-273.
- ^ Luger SW, Paparone P, Wormser GP, Nadelman RB, Grunwaldt E, Gomez G, Wisniewski M, Collins JJ. Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans. Antimicrobial Agents and Chemotherapy. March 1995, 39 (3): 661–7. PMC 162601 . PMID 7793869. doi:10.1128/AAC.39.3.661.
- ^ Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, Welch P, Marcus R, Agüero-Rosenfeld ME, Dennis DT, Wormser GP. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. The New England Journal of Medicine. July 2001, 345 (2): 79–84. PMID 11450675. doi:10.1056/NEJM200107123450201 . 已忽略未知参数
|df=(帮助) - ^ Karlsson M, Hammers-Berggren S, Lindquist L, Stiernstedt G, Svenungsson B. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology. July 1994, 44 (7): 1203–7. PMID 8035916. S2CID 38661885. doi:10.1212/WNL.44.7.1203.
- ^ Weinstein RS. Human ehrlichiosis. American Family Physician. November 1996, 54 (6): 1971–6. PMID 8900357.
- ^ Karlsson U, Bjöersdorff A, Massung RF, Christensson B. Human granulocytic ehrlichiosis--a clinical case in Scandinavia. Scandinavian Journal of Infectious Diseases. 2001, 33 (1): 73–4. PMID 11234985. S2CID 218880245. doi:10.1080/003655401750064130.
- ^ 29.00 29.01 29.02 29.03 29.04 29.05 29.06 29.07 29.08 29.09 29.10 29.11 29.12 29.13 29.14 29.15 29.16 29.17 29.18 29.19 29.20 29.21 29.22 29.23 29.24 29.25 29.26 29.27 29.28 29.29 29.30 29.31 29.32 Doxycycline, ANDA no. 065055 Label. (PDF). U.S. Food and Drug Administration. 2012-12-14. (原始内容 (PDF)存档于2014-04-19).
- ^ 30.00 30.01 30.02 30.03 30.04 30.05 30.06 30.07 30.08 30.09 30.10 30.11 30.12 30.13 30.14 30.15 30.16 30.17 30.18 30.19 30.20 30.21 30.22 30.23 30.24 30.25 30.26 30.27 30.28 30.29 30.30 30.31 Doxycycline, ANDA no. 065454 Label (PDF). U.S. Food and Drug Administration. 2008-07-16. (原始内容 (PDF)存档于2013-10-19).
- ^ Gupta N, Boodman C, Jouego CG, Van Den Broucke S. Doxycycline vs azithromycin in patients with scrub typhus: a systematic review of literature and meta-analysis. BMC Infect Dis. December 2023, 23 (1): 884. PMC 10726538 . PMID 38110855. doi:10.1186/s12879-023-08893-7 .
- ^ Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, Nicholson WL, Paddock CD, Sexton DJ. Diagnosis and management of Q fever--United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR. Recommendations and Reports. March 2013, 62 (RR-03): 1–30. PMID 23535757. (原始内容存档于2014-04-19). 已忽略未知参数
|df=(帮助) - ^ Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, Folk SM, Kato CY, Lash RR, Levin ML, Massung RF, Nadelman RB, Nicholson WL, Paddock CD, Pritt BS, Traeger MS. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis — United States. MMWR. Recommendations and Reports. 2016, 65 (2): 1–44 [2024-02-05]. PMID 27172113. doi:10.15585/mmwr.rr6502a1 . (原始内容存档于2023-01-28).
- ^ Schutze GE, Regan J, Bradley J. Use doxycycline as first-line treatment for rickettsial diseases. AAP News. American Academy of Pediatrics. 2010-07-01 [2024-02-05]. ISSN 1556-3332. (原始内容存档于2024-02-05).
- ^ Spotted fever group rickettsial disease | DermNet. 2023-10-26 [2024-02-05]. (原始内容存档于2024-02-05).
- ^ Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Chiba N, Iwata S, Ubukata K. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clinical Infectious Diseases. December 2012, 55 (12): 1642–9. PMID 22972867. doi:10.1093/cid/cis784.
- ^ Lyme disease. Treatment. 2018-12-21. (原始内容存档于2016-06-10).
- ^ Taylor-Salmon E, Shapiro ED. Tick-borne infections in children in North America. Curr Opin Pediatr. April 2024, 36 (2): 156–163. PMC 10932821 . PMID 38167816. doi:10.1097/MOP.0000000000001326. 已忽略未知参数
|pmc-embargo-date=(帮助) - ^ Dreno B, Thiboutot D, Gollnick H, Bettoli V, Kang S, Leyden JJ, Shalita A, Torres V. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. European Journal of Dermatology. 2014, 24 (3): 330–4. PMID 24721547. S2CID 28700961. doi:10.1684/ejd.2014.2309.
- ^ Garner SE, Eady A, Bennett C, Newton JN, Thomas K, Popescu CM. Minocycline for acne vulgaris: efficacy and safety. The Cochrane Database of Systematic Reviews. August 2012, 2012 (8): CD002086. PMC 7017847 . PMID 22895927. doi:10.1002/14651858.CD002086.pub2. 已忽略未知参数
|collaboration=(帮助) - ^ van Zuuren EJ, Fedorowicz Z, Carter B, van der Linden MM, Charland L. Interventions for rosacea. The Cochrane Database of Systematic Reviews. April 2015, 2015 (4): CD003262. PMC 6481562 . PMID 25919144. doi:10.1002/14651858.CD003262.pub5. 已忽略未知参数
|collaboration=(帮助) - ^ Cao H, Yang G, Wang Y, Liu JP, Smith CA, Luo H, Liu Y. Complementary therapies for acne vulgaris. The Cochrane Database of Systematic Reviews. January 2015, 1 (1): CD009436. PMC 4486007 . PMID 25597924. doi:10.1002/14651858.CD009436.pub2. 已忽略未知参数
|collaboration=(帮助) - ^ 43.0 43.1 Doxycycline Dosage Guide + Max Dose, Adjustments. [2024-02-05]. (原始内容存档于2024-02-05).
- ^ Vanbaelen T, Manoharan-Basil SS, Kenyon C. 45 years of tetracycline post exposure prophylaxis for STIs and the risk of tetracycline resistance: a systematic review and meta-analysis. BMC Infect Dis. April 2024, 24 (1): 376. PMC 10996150 . PMID 38575877. doi:10.1186/s12879-024-09275-3 .
- ^ Samuel K. Using antibiotics to prevent STIs. Aidsmap. 2023-05-26 [2024-03-10]. (原始内容存档于2024-03-10).
- ^ Stewart J. Doxycycline Prophylaxis to Prevent Sexually Transmitted Infections in Women. New England Journal of Medicine. 2023-12-21, 389 (25): 2331–2340. PMC 10805625 . PMID 38118022. doi:10.1056/NEJMoa2304007.
- ^ Cornelisse VJ, Riley B, Medland NA. Australian consensus statement on doxycycline post-exposure prophylaxis (doxy-PEP) for the prevention of syphilis, chlamydia and gonorrhoea among gay, bisexual and other men who have sex with men. Med J Aust. April 2024, 220 (7): 381–386. PMID 38479437. doi:10.5694/mja2.52258 .
- ^ Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial STI Prevention. CDC. 2023-09-29 [2024-03-10]. (原始内容存档于2024-03-10).
- ^ 2023 Consensus Statement on doxycycline prophylaxis (Doxy-PEP) for the prevention of syphilis, chlamydia and gonorrhoea among gay, bisexual, and other men who have sex with men in Australia. Australasian Society for HIV Medicine. [10 March 2024]. (原始内容存档于2024-03-10).
- ^ Highleyman L. Sexually transmitted infections in San Francisco have fallen since doxyPEP roll-out. Aidsmap. 5 March 2024 [2024-03-10]. (原始内容存档于2024-03-10).
- ^ Hashemi SH, Gachkar L, Keramat F, Mamani M, Hajilooi M, Janbakhsh A, Majzoobi MM, Mahjub H. Comparison of doxycycline-streptomycin, doxycycline-rifampin, and ofloxacin-rifampin in the treatment of brucellosis: a randomized clinical trial. International Journal of Infectious Diseases. April 2012, 16 (4): e247–e251 [2014-08-23]. PMID 22296864. doi:10.1016/j.ijid.2011.12.003 . (原始内容存档于2021-08-28).
- ^ Doryx- doxycycline hyclate tablet, delayed release. DailyMed. 2020-10-23 [2022-03-05]. (原始内容存档于2022-01-03).
- ^ Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrobial Agents and Chemotherapy. September 2006, 50 (9): 3124–31. PMC 1563505 . PMID 16940111. doi:10.1128/AAC.00394-06.
- ^ Holmes NE, Charles PG. Safety and Efficacy Review of Doxycycline. Clinical Medicine. Therapeutics. 2009, 1: CMT.S2035. doi:10.4137/CMT.S2035.
- ^ Gaillard T, Madamet M, Pradines B. Tetracyclines in malaria. Malar J. November 2015, 14: 445. PMC 4641395 . PMID 26555664. doi:10.1186/s12936-015-0980-0 .
- ^ Guidelines for the treatment of malaria. Geneva: World Health Organization. 2015: 246. ISBN 978-92-4-154912-7.
- ^ Hoerauf A, Mand S, Fischer K, Kruppa T, Marfo-Debrekyei Y, Debrah AY, Pfarr KM, Adjei O, Büttner DW. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Medical Microbiology and Immunology. November 2003, 192 (4): 211–6. PMID 12684759. S2CID 23349595. doi:10.1007/s00430-002-0174-6.
- ^ Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial. Lancet. 2005, 365 (9477): 2116–21. PMID 15964448. S2CID 21382828. doi:10.1016/S0140-6736(05)66591-9.
- ^ Kaufman JA, Lee MJ. Vascular and interventional radiology 2nd. Philadelphia, PA: Saunders. 22 June 2013. ISBN 978-0-323-07672-2. OCLC 853455295.
- ^ Müller M, Butler J, Heidecker B. Emerging therapies in transthyretin amyloidosis – a new wave of hope after years of stagnancy?. European Journal of Heart Failure (Wiley). 7 January 2020, 22 (1): 39–53. PMID 31912620. doi:10.1002/ejhf.1695 .
- ^ 61.0 61.1 Haberfeld H (编). Austria-Codex. Vienna: Österreichischer Apothekerverlag. 2020. Doxycyclin Genericon 200 mg lösliche Tabletten (德语).
- ^ ((PubMed Health)). Doxycycline (By mouth). U.S. National Library of Medicine. 2016-07-01 [2016-07-16]. (原始内容存档于2013-11-11).
- ^ Chung AM, Reed MD, Blumer JL. Antibiotics and breast-feeding: a critical review of the literature. Paediatric Drugs. 2002, 4 (12): 817–37. PMID 12431134. S2CID 8595370. doi:10.2165/00128072-200204120-00006.
- ^ Mylonas I. Antibiotic chemotherapy during pregnancy and lactation period: aspects for consideration. Archives of Gynecology and Obstetrics. January 2011, 283 (1): 7–18. PMID 20814687. S2CID 25492353. doi:10.1007/s00404-010-1646-3.
- ^ 65.0 65.1 Side effects of doxycycline. NHSP. [2024-11-11].
- ^ Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: a myth debunked. Journal of the American Academy of Dermatology. June 2002, 46 (6): 917–23. PMID 12063491. doi:10.1067/mjd.2002.120448.
- ^ Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H. European recommendations on the use of oral antibiotics for acne (PDF). European Journal of Dermatology. November–December 2004, 14 (6): 391–9. PMID 15564203.[永久失效链接]
- ^ DeRossi SS, Hersh EV. Antibiotics and oral contraceptives. Dental Clinics of North America. October 2002, 46 (4): 653–64. CiteSeerX 10.1.1.620.9933 . PMID 12436822. doi:10.1016/S0011-8532(02)00017-4.
- ^ Flower R, Rang HP, Dale MM, Ritter JM, Henderson G. Rang & Dale's Pharmacology. Edinburgh: Churchill Livingstone. 2012. ISBN 978-0-7020-3471-8.
- ^ Hitchings A, Lonsdale D, Burrage D, Baker E. Top 100 drugs: clinical pharmacology and practical prescribing. Churchill Livingstone. 2015: 200–201. ISBN 978-0-7020-5516-4.
- ^ Maaland MG, Papich MG, Turnidge J, Guardabassi L. Pharmacodynamics of doxycycline and tetracycline against Staphylococcus pseudintermedius: proposal of canine-specific breakpoints for doxycycline. Journal of Clinical Microbiology. November 2013, 51 (11): 3547–54. PMC 3889732 . PMID 23966509. doi:10.1128/JCM.01498-13.
- ^ Doxycycline. www.drugbank.ca. [2019-01-23]. (原始内容存档于2008-11-10).
- ^ Pfizer, Inc. v. International Rectifier Corp., 545 F. Supp. 486 (C.D. Cal. 1980). Justia Law. (原始内容存档于2015-02-24).
- ^ Pfizer to Get Rachelle Units. The New York Times. The Associated Press. 1983-07-06. (原始内容存档于2016-03-06).
- ^ Nationwide Shortage of Doxycycline: Resources for Providers and Recommendations for Patient Care. CDC Health Alert Network. 2013-06-12. (原始内容存档于2015-02-15).
- ^ Sudden increase in cost of common drug concerns many. WSMV-TV. 2013-03-12. (原始内容存档于2014-12-31).
- ^ Rosenthal E. Officials Question the Rising Costs of Generic Drugs. The New York Times. 2014-10-07. (原始内容存档于2017-02-23).
- ^ Palmer E. Hikma hits the jackpot with doxycycline shortage. FiercePharmaManufacturing. 2014-03-13. (原始内容存档于2015-01-01).
- ^ Costco Drug Information. [2016-07-31]. (原始内容存档于2016-03-04).
- ^ Doxycycline Hyclate Prices and Doxycycline Hyclate Coupons. GoodRx. [2016-07-31]. (原始内容存档于2016-07-28).
- ^ International availability for doxycycline. drugs.com. [2015-04-29]. (原始内容存档于2015-05-16).
- ^ Leung E, Landa G. Update on current and future novel therapies for dry age-related macular degeneration. Expert Review of Clinical Pharmacology. September 2013, 6 (5): 565–79. PMID 23971874. S2CID 26680094. doi:10.1586/17512433.2013.829645.
- ^ Greenwald RA. The road forward: the scientific basis for tetracycline treatment of arthritic disorders. Pharmacological Research. December 2011, 64 (6): 610–3. PMID 21723947. doi:10.1016/j.phrs.2011.06.010.
- ^ Krakauer, Teresa; Buckley, Marilyn. Doxycycline Is Anti-Inflammatory and Inhibits Staphylococcal Exotoxin-Induced Cytokines and Chemokines. Antimicrobial Agents and Chemotherapy. November 2003, 47 (11): 3630–3633 [2024-11-11]. doi:10.1128/AAC.47.11.3630-3633.2003.
- ^ Saliy O, Popova M, Tarasenko H, Getalo O. Development strategy of novel drug formulations for the delivery of doxycycline in the treatment of wounds of various etiologies. Eur J Pharm Sci. April 2024, 195: 106636. PMID 38185273. doi:10.1016/j.ejps.2023.106636 .
- ^ Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. American Journal of Ophthalmology. July 2001, 132 (1): 8–13. PMID 11438047. doi:10.1016/S0002-9394(01)00913-8.